Abstract
This study focused on determining the influence of temperature (500–700 °C) during pyrolysis of pelletised chicken litter (PCL) and fresh chicken litter (FCL). The composition of all pyrolysis products was analysed, and their potential applications were discussed. An analysis of phosphorus speciation in FCL and PCL along with their derived biochars revealed that the share of water-soluble phosphorus was greatly reduced in the biochar, implying lower risk of eutrophication in agricultural applications of biochar when used as a soil improver. Indeed, water-soluble phosphorus decreased from 60% for PCL to as low as 3% for the biochars. In addition, the concentration of other nutrients and heavy metals in biochar, and its potential for agriculture application was discussed. Heavy metals content was below the upper limits set out in the European Fertilising Products Regulation only for biochars produced at 500 °C, but biochars produced at higher temperatures did not meet the limits for Zn and Ni content. The energy balance analysis showed that pelletisation of chicken litter is not necessary, as the properties of both PCL and FCL allow for energetically sustainable pyrolysis when hot pyrolysis gas is combusted, and biochar recovered for nutrient recycling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Poultry meat dominates the meat market share over beef since the outbreak of Bovine spongiform encephalopathy (also known as mad cow disease) in 1974. In 2020, 1.06 billion broilers, 62 million fowl, and 16 million turkeys were slaughtered in the UK alone [1]. However, increasing demand for poultry meat led the transition of poultry farming from extensive to intensive. Although intensive farming succeeded in addressing the market demand for poultry meat nevertheless, it poses a significant threat to the environment if the farming waste is not disposed of sustainably [2, 3]. The Food and Agriculture Organisation (FAO) of the United Nations estimated that global livestock production currently accounts for 15% of the total anthropogenic greenhouse gas (GHG) emissions (equivalent to 7.1 giga tonnes of CO2eq per year) [4]. Accumulation of animal manure brings a massive disposal problem; therefore, sustainable treatment technologies are imperative to avoid adverse impacts on the environment [5]. Land application and composting of chicken litter are common and are the cheapest methods of managing and disposing of it in large quantities [6]. However, due to stricter environmental regulations, the proportion of soil application and composting treatment facilities is gradually declining. Moreover, sustainable disposal opportunities exist to utilise the animal waste on farms to generate heat, power, and fertiliser via a thermochemical conversion route which can contribute to economic sustainability. Chicken litter has been proposed and utilised as a fuel and/or fertiliser [6, 7].
Pyrolysis has been considered an alternative technology to direct incineration with added technical and environmental advantages. Pyrolysis processes can reduce the solid waste to 80–95% by volume, while being converted into liquid, solid, and gaseous fractions, namely bio-oil (liquid product containing the aqueous phase and the organic phase, pyrolysis oil) , biochar, and pyrolysis gas [8]. Pyrolysis processes are mainly divided into two categories: slow and fast pyrolysis. Slow pyrolysis occurs at low heating rates (25–120 °C/min) and long residence times (15–36 min) where biochar is the main product [9, 10]. In contrast, fast pyrolysis takes place at higher heating rates (600 °C/s) shorter residence times (0.5–5 s) and produces a higher yield of liquid and non-condensable gases [11, 12]. However, it is evident from Table 1 that for both slow and fast pyrolysis of chicken manure, biochar is the main product due to the high ash content, which is a significant constituent of chicken manure (14–22 wt. %) and derived biochar (42–66 wt. %). The high ash content reduces contribution of lignocellulosic biomass (bedding material) in chicken manure, main precursor of liquid product. Since slow pyrolysis generates higher biochar yield, it is generally more suitable to convert agricultural waste into biochar [13]. The inorganic compounds mostly accumulate in the biochar which makes it high-quality soil improver. It has been estimated that the pyrolysis gas produced can sustain the energy demand for continuous operation of the process [14]. This opens the prospect of slow pyrolysis as a disinfection treatment of bio-hazardous materials as well as waste-to-energy utilisation of chicken litter [9].
Previous research work on the slow and fast pyrolysis of chicken litter and their comparative yield analyses are presented in Table 1.
According to Elliot [16], primary pyrolysis oil contains a mixture of oxygenates and phenolic ethers at pyrolysis temperature of 400 and 500 °C. The phenolic compounds are formed by the decomposition of lignin, while different oxygenates, for example, sugars and furans, are produced by the degradation of cellulose and hemicellulose in the feedstock; in chicken litter, bedding material is a source of these. Esters, acids, alcohols, ketones, and aldehydes derive from decomposition and reformation of random small, oxygenated compounds [17]. The formation of nitrogen-containing compounds (i.e. pyrazines, pyridines) was attributed to the pyrolysis of proteins and peptides in the poultry litter feedstock [15, 18].
Current research studies mainly focused on utilisation of pyrolysis liquid products in biotechnological applications. Arnold et al. [19] reviewed processes that use pyrolysis oil as a substrate for industrially relevant bioproduction employing microbiological strains. There are three main challenges for the biotechnological conversion of this carbon source: (i) general toxicity, (ii) an overall complex composition, and (iii) a low concentration of bio-accessible molecules. Moita and Lemos [20] used the entire pyrolysis oil obtained by fast pyrolysis of residues from poultry farming (chicken beds) as a possible microbial feedstock for the production of short-chain polyhydroxyalkanoates (i.e. bio-plastics) using mixed microbial cultures. Another study showed that after phase separation, the organic phase could potentially be used for direct combustion as a boiler fuel [21]. However, to assess its suitability, additional properties such as acidity, solid and ash content, and water content need to be determined [22].
The oil from fast pyrolysis of poultry litter did not comply with requirements for biodiesel engines due to the high water content (26 wt.%) that inhibits ignition (upper limit 0.5 wt.%) and very high total acidity number of 38–46 mg of KOH/g (upper limit 0.5 mg of KOH/g). The total amount of inorganic trace elements (ash) in the poultry litter pyrolysis oil also exceeded the upper acceptable limit (0.02 wt.%) by tenfold [15].
The only current state-of-the-art application of hot pyrolysis vapour is direct combustion with recovery of energy from the flue gas in a micro-gas turbine [23, 24].
Production of biochar from chicken litter is of increased scientific interest due to the soil improving porous structure and nutrient content of biochar [25, 26] as well as using biochar in carbon sequestration for alleviating the effects of global warming. Recently, several researchers showed that biochar produced at temperatures between 300 and 450 °C is suitable for agricultural applications [26,27,28,29,30]. Biochar derived from poultry manure (obtained at 460 °C) contains phosphorus chemical species with good plant availability making it suitable as a slow release or phosphorus storage fertiliser in slightly acidic soils [31]. Furthermore, another study showed that a low pyrolysis temperature (350–500 °C) had a more positive effect on nutrient availability compared with combustion (at 815 °C), and it is a suitable method for the thermal treatment of chicken manure [32]. Phosphorus solubility and related availability to plants depends on several factors, one of which is the mineralogical phase binding of nutrient [32]. It has been reported that inorganic orthophosphate is available to plants [33, 34]. Apatite-bound phosphorus is not directly available to plants; however, recent research shows that it is not recalcitrant either but partially available to plants, even within one cropping season, but the degree of its utilisation depends on the overall nutrient status and soil water distribution [35]. The forms of phosphorus in manure could be broadly classified as organically associated phosphorus and inorganic phosphorus [36]. For poultry litter, phosphorus is present in the form of organically associated phytate and phospholipid-type species.
So far, most studies on agricultural application of poultry/chicken litter biochar focus on macronutrients (such as N-P-K, Ca, Mg) availability, as well as emissions of NH3, NO, etc. but did not investigate the content of heavy metals in chicken/poultry litter biochar. Poultry/chicken litter has a high concentration of Zn and Cu, typical supplements in poultry diet which affects bird’s growth performance [37]. These elements are often added to poultry/chicken feed in larger amounts than the recommended daily dose because they do not absorb easily [38]. The content of Zn and Cu is increased following thermal processing of poultry litter, e.g. combustion [39], gasification [40], and pyrolysis, due to a concentration effect.
The development of circular economy aims to prevent uncontrolled dispersal of plant nutrients to mitigate environmental damage [41]. The application of chicken/poultry litter to arable land in regions with intensive farming is becoming unsustainable on the one hand, due to phosphorus accumulation and uncontrolled phosphorus losses (water-soluble phosphorus leaks to water streams causing eutrophication), and on the other hand GHG emissions [4]. Pyrolysis technology and biochar production can be economically viable and consistent with the UN’s sustainability goals and the circular economy principles when using locally available feedstock that is processed at local pyrolysis plants [42, 43].
The new European Fertilising Products Regulation (FPR) [44] allows fertilising products based on organic or recovered materials. The European Commission is currently carrying out a survey on the possible inclusion of STRUBIAS materials, such as struvite, biochar, and ashes, which do not present a risk to human, animal, or plant health and are safe for the environment. In the new FPR [44], animal by-products (manures) are authorised as input materials for EU fertilising products such as pyrolysis materials (biochar).
A recent study investigated the speciation, plant availability, and environmental risk of heavy metals (Zn, Cu, Ni, Mn, Cr, As, Cd) in chicken manure biochar produced at temperatures from 200 to 800 °C. Based on BCR (Bureau Communautaire de Reference) sequential extraction [45, 46]), it was concluded that the percent of acid-extractable and reducible portion of heavy metals declined and the percent of the residual portion (non-bioavailable and non-toxic) increased with temperature [47]. Additionally, the toxicity characteristic leaching procedure showed that the leaching of heavy metals decreases with increasing pyrolysis temperature as does the concentration of plant available heavy metals. In contrast, chicken manure biochar (prepared at 500 °C) immobilised Cd and Pb in contaminated soil during accelerated ageing [48].
Baniasadi et al. investigated slow pyrolysis and demonstrated that the energy transferred to the non-condensable gases was sufficient to thermally self-sustain the pyrolysis process at 550 °C provided the moisture content of poultry litter does not exceed 15 wt.% [9]. The study on intermediate pyrolysis of chicken manure with a moisture content of 10 wt.%, showed that hot pyrolysis gas may be directly combusted to produce electricity in a micro-gas turbine and heat for internal utilisation (on site use) [24]. However, when nitrogen was used as a carrier gas during pyrolysis of biomass waste and heating of the carrier gas was included in the energy balance, an additional 4–10 % of HHV of the feedstock was required to achieve energetically self-sustaining intermediate pyrolysis [49]. It was shown in a previous study [50] that there is no need to use nitrogen as a carrier gas for production of engineered biochars.
Previously conducted research indicated that intermediate pyrolysis is a suitable technology for decentralised production of biochar in small scale facilities such as chicken/poultry farms [24]. In this study, the pyrolysis regime took place between slow and fast pyrolysis, referred to as intermediate pyrolysis with a residence time of about 10 min [51]. Such a residence time is sufficiently long to allow full carbonisation of manures with minimal energy requirements. To the best of authors’ knowledge, there are no previous studies available comparing the intermediate pyrolysis of fresh and pelletised chicken litter. Our study also assessed the potential application of the biochar produced and provided detailed analysis of the biochar including the content of plant nutrients and heavy metals. An attempt was made to explain the fate of phosphorous in chicken litter. The overarching goal of this study is to investigate the influence of pyrolysis temperature on conversion of raw and pelletised chicken litter into useful energy resources by means of intermediate pyrolysis as well as a potential application of biochar in agriculture. The specific objectives of this study are (1) investigating the effect of pelletisation, (2) investigating the effect of temperature; (3) investigating nutrient availability and innocuity of biochar; and (4) evaluating the mass and energy balance of intermediate pyrolysis process.
2 Experimental section
2.1 Feedstock
Two types of chicken litter were used as feedstock: fresh chicken litter (FCL) and pelletised chicken litter (PCL). FCL was obtained from a local Irish chicken farm, whereas PCL was collected from a Finnish chicken farm. PCL was sieved, partially dried, and pelletised into approx. 0.5 cm diameter and 1 cm long pellets and was supplied by Biolan, Finland (Fig. S-1 in supplementary information). Chicken litter is a heterogeneous feedstock; hence, its composition depends on various factors such as animal feed, bedding material, feathers, excreta, and moisture content [6]. Ultimate and proximate properties of both feedstock and biochars produced along with their heating values are presented in Table 2.
Since PCL was partially dried, the reported moisture content in FCL was much higher than PCL, 22.7 versus 4.8 wt.%. However, the ash content in FCL 9.8 wt.% is almost half of that in PCL, 17 wt.%. It is evident from Table 2 that PCL has higher C and H content because peat was used as a bedding material rather than Sitka spruce (Picea sitchensis) which corresponds to the higher calorific value of PCL compared to FCL.
The main inorganic constituent found in FCL and PCL (Table S-1) was potassium (K) 25400 and 37900 mg/kgdry matter followed by phosphorous (P) and calcium (Ca). Concentrations of phosphorus were similar in both feedstocks 10583 and 14397 mg/kgdry matter. The calcium content of 18800 mg/kgdry matter in PCL was almost four times higher than in FCL (4985 mg/kgdry matter).
2.2 Experimental procedure
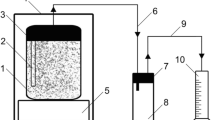
Pyrolysis experiments were conducted in the Department of Chemical Sciences, University of Limerick, Ireland. Figure 1 shows the schematic diagram of pyrolysis apparatus. The experimental set-up consisted of a quartz glass tube reactor and a borosilicate glass condensation unit. The internal diameter of the reactor was 45 mm, while the length was 600 mm. The set temperature was achieved and maintained by heating tape (Omegalux, USA) with a maximum power output of 1254 watts, and heating tape was further insulated with glass-fibre tape. The temperature regime in the reactor was controlled by using an electrothermal regulator (Cole-Parmer, UK). Ethylene glycol was used as a heat transfer medium in the condensation unit and maintained at −5 °C throughout the experiments. Raw pyrolysis gas from the reactor was cooled down while passing through the condensation unit. A twin-neck flask was used to collect the pyrolysis liquids. The outlet of the twin-neck flask was connected to a filter (filled with loosely packed paper tissue) to capture remaining aerosols. When the pyrolysis gas was not collected for analysis, it was released in the fume hood.
Before an experiment, the pyrolysis reactor was flushed with nitrogen for 5 min; then, the heating of the reactor tube started with the rubber stopper on. Once reactor reached the set temperature (500, 600, or 700°C), the stopper together with the thermocouple was removed to allow insertion of a metal basket filled with 50 g of feedstock; then, the reactor was closed again. No nitrogen flow was applied after sample loading, but a small amount of air entered the reactor during sample insertion activity, 2 to 10 vol. % of N2 and up to 2 vol. % of O2, was typically measured in the pyrolysis gas. The pyrolysis process terminated when no visual vapours were observed in the reactor tube. This occurred within 10 to 12 min. After termination of the pyrolysis process, the biochar was allowed to cool down in the reactor. Each experiment was repeated five times to collect data for mass balance calculation. Pyrolysis gas samples from two out of five experiments were collected, and an average gas composition was calculated. For pyrolysis gas collection, a Tedlar bag was connected directly to the outlet of the twin-neck receiving flask after the apparatus was flushed with nitrogen. After completing all repetitions, the apparatus was disconnected and weighed. The relative distribution of pyrolysis products was determined by the principle of conservation of mass i.e. the combined mass of pyrolysis products is equal to that of the initial mass of the feedstock. The initial feedstock mass and the biochar mass were measured directly before and after the pyrolysis experiment, respectively, and the biochar yield was calculated. The mass of pyrolysis liquids (oil and aqueous condensable fraction) was calculated as the difference in the mass of the apparatus (reactor with all associated components) before and after a set of pyrolysis tests at the same temperature (five tests). The mass fraction of pyrolysis gas was calculated as the difference between initial mass of feedstock, minus biochar, and liquid mass.
The following scenario was assessed with regard to the end use of the pyrolysis products: biochar was considered as a soil amendment whereas the hot pyrolysis gas (non-condensable gases and condensable organic fraction together with water-soluble compounds) was combusted to provide heat for drying and pyrolysis. The energy input with the feedstock (QINPUT) was calculated by multiplying the HHV of FCL/PCL and the mass of 1 kg of FCL/PCL as received. The energy output of the pyrolysis biochars (QBIOCHAR) was calculated from the HHV and biochar yield (Table 3). The energy recovered in the hot pyrolysis vapours (QGAS) was calculated as the difference between QINPUT and QBIOCHAR. When calculating the available thermal energy released, Qavail, from the combustion of the hot pyrolysis gases, a combustion efficiency of 80 % was assumed. The ratio Qavail/Qtotal ≥ 1 indicated that the pyrolysis process is energetically sustainable.
2.3 Analytical techniques
The solid samples were characterised by proximate and ultimate analysis. The proximate analysis (moisture content, volatile matter, ash, and fixed carbon content) was determined according to British standards [52,53,54]. The ultimate analysis (the content of C, H, N, S, and O) was determined using an elemental analyser (Vario EL Cube). Higher heating value (HHV) of solid samples was measured using a bomb calorimeter (Parr 6200 Isoperibol) and followed by chlorine (Cl) measurement according to standard protocol [55]. For determination of inorganic constituents, FCL, PCL, and biochars were ashed at 550 °C, then 0.2 g of sample ash was dissolved with 6 ml of nitric acid, 4 ml of hydrogen peroxide, and 4 ml of hydrofluoric acid at 190 °C in a microwave digestion oven (Mars 6). After complexation with boric acid, the content of the main and trace elements (Al, Ca, Fe, K, Mg, Na, P, S, Si, Ag, As, Ba, Cd, Co, Cr, Cu, Hg, Mn, Mo, Ni, Pb, Sb, Se, Ti, Sn, V, Zn) was measured using inductively coupled plasma optical emission spectrometry (Agilent 5100 ICP-OES fitted with an SPS4 auto-sampler). ICP-OES instrument was calibrated against four concentration levels.
Pyrolysis gas samples were analysed using gas chromatograph Agilent 3000, which enables identification and quantification of gas compounds CO2, CO, C2H4, C2H6, CH4, H2S, H2, N2, and O2. The results were presented on a N2 and O2 free basis which allows direct comparison of gas composition between two different feedstock and temperatures while avoiding any dilution effect due to the presence of a small amount of air in the apparatus as a result of sample loading into the reactor. The HHV of the combustible pyrolysis gas was calculated based on the volumetric fraction (f) of specific gas compounds and their respective HHV [8] using Eq. (1):
To convert HHV of pyrolysis gas from MJ/m3 to MJ/kg, the HHV was divided by the density of pyrolysis gas.
The release of nitrogen and sulphur-containing compounds and other pollutants during the pyrolysis of chicken litter is of fundamental relevance for further treatment of pyrolysis gas/vapours (non-condensable gases and condensable compounds). It is worth mentioning that in this study the content of NH3 in pyrolysis gas was not measured directly, but the nitrogen-containing organic compounds were detected in liquid products (Tables S-2 to S-4).
The HHV of liquid product was calculated based on mass and energy conservation principles. Mass and energy contained in feedstock equals mass and energy of all pyrolysis products combined [10]. Calculations were carried out on an as received basis (ar).
The Karl Fisher titration was used to determine the moisture content in the liquid pyrolysis product. The moisture content was used to calculate the yield of aqueous and oil fractions from the total liquid pyrolysis product. An Agilent 7890A GC, coupled with a triple-axis MSD 5975C (GC-MSD), was used to separate and identify organic compounds. Prior to the injection into the GC-MSD, the pyrolysis liquid was 20 × diluted in isopropanol to avoid column or detector overload by either matrix or analyte. Isopropanol simultaneously dissolves aromatic hydrophobic bio-oil compounds and water-soluble hydrocarbons into one homogeneous solution. Helium was used as the carrier gas. The flow rate of helium through the capillary column HP-5ms (30 m × 250 μm × 0.25 μm) was set to 1.6 mL/min. The injection port kept at 300 °C received 1 μL of a manually injected sample. The injection was conducted in a split (10:1) mode. The oven temperature programme initiated at 60 °C for 7 min and was increased to 180 °C at a rate of 3 °C/min, with a final ramp from 180 to 260 °C at a rate of 4 °C/min, making up a total run time of 67 min. The mass spectrometer (i.e. MSD) operated in a full scan mode in the mass range of 40−550 m/z. Solvent delay time was adjusted to 1.2 min, while the gain factor setting was 1. Electron ionisation energy was set to 70 eV. The transfer line, MSD ion source, and MSD quadrupole mass analyser temperatures were maintained at 300, 180, and 150 °C, respectively. The performance of the MSD was checked daily using the auto tune function. Total ion current chromatograms between retention times of 1.30 min and 67.00 min were integrated (by MassHunter Qualitative Analysis B.07.00 software). After chromatographic separation and identification, the most abundant bio-oil compounds were quantified by employing external standard calibration. The calibration curve was plotted against four concentration levels of phenol. A single calibration curve is a simplified approach to quantify several tens of distinct heterocyclic compounds present in the bio-oil. However, the response of the MSD is affected by the chemical structure of the compounds [56]. Phenol, as an external standard, was selected due to its abundance in the bio-oil and because it mimics the chemical structure of other compounds present in the pyrolysis oil.
Aqueous extracts of phosphorus (water-soluble P) were obtained by shaking ∼0.5 g of FCL, PCL, and biochars with 40 mL of distilled water at room temperature for 24 h in an orbital shaker (IKA 130 Basic) set to 200 rpm. The suspension was then centrifuged and filtered with a 0.45-μm syringe filter. The phosphorus content in the extract was determined by ICP-OES.
The different chemical forms of phosphorus in FCL, PCL, and biochars were determined following the extraction using HCl and NaOH as extractants [57, 58]. The protocol, which includes three separate extraction procedures, was originally designed to obtain five phosphorus fractions. The first procedure was used for the determination of total phosphorus (total P) fraction as an overall indicator; the second procedure was used for the inorganic phosphorus fraction (inorganic P), which is mainly labile phosphorus (weakly bound to the sample matrix) and the organic phosphorus fraction (organic P). The third procedure was used for the apatite phosphorus fraction (apatite P), which is a stable form of phosphorus and assumed to be associated with Ca, and the non-apatite inorganic phosphorus fraction (non-apatite inorganic P), which is moderately labile phosphorus and assumed to be associated with oxides and hydroxides of Al, Fe, and Mn, respectively [58]. According to this protocol, the total P includes organic P and inorganic P. Inorganic P includes apatite P and non-apatite inorganic P. A detailed description of the extraction procedure can be found in [59]. The content of phosphorus in all extracts was measured by ICP-OES.
3 Results and discussion
3.1 Product yields
The product yield distribution for the two types of chicken litter pyrolysed at different temperatures is shown in Table 3. The distribution was expressed as mass percent taking initial feedstock mass as a reference point. The product yield distribution pattern for both feedstocks was similar; however, more pronounced differences in product yield at different temperatures were observed for FCL, especially for the liquid and gaseous fractions. Biochar yield decreased with increasing temperature, from around 36 to 23 wt.% for FCL and from around 38 to 30 wt.% for PCL. The biochar yield was lower for FCL. The yield of liquid fraction at 500 and 600 °C was significantly higher for FCL which is due to the higher initial moisture content. However, this effect was not observed at the highest pyrolysis temperature 700 °C. At this temperature, rapid water evaporation causes a sharp increase in local pressure ripping the sample structure from the inside. These phenomena promoted devolatilisation and resulted in a decrease of biochar yield, while increasing the overall pyrolysis gas yield. A steam rich atmosphere also favours steam reforming reactions of primary devolatilisation products. The effect of moisture content is reflected in the reduction of pyrolysis oil (see Table 4), while the yield of hydrogen rich gas increased as was reported previously [60, 61]. It can be observed that at 700 °C the gas yield from FCL reached 45 wt.% of initial feedstock mass and it was higher than the gas yield from PCL.
A comparison of experimental results from this study with results for intermediate pyrolysis from literature is presented in Table 4. The yields of PCL biochar and pyrolysis gas produced at 500 °C were comparable with the yields obtained at the same temperature measured by Morgano et al. [24] for pelletised chicken manure. However, the yields of pyrolysis oil and aqueous phase, primarily affected by the initial composition of chicken litter such as type of bedding material and moisture content, were different.
3.2 Mass balance analysis
The content of C, H, and N in biochars decreased with an increase in pyrolysis temperature since these elements were released as gaseous compounds (non-condensable or condensable) (Table 2). Mass balance calculations revealed that for FCL 25.7, 14.5, and 9.9 wt.% and for PCL 22.2, 19.1, and 13.8 wt.% of the initial N was retained in the biochars for pyrolysis process conducted at the temperatures of 500, 600, and 700 °C, respectively. Nitrogen is a primary plant nutrient; therefore, high N content in biochar for agricultural applications is desirable. A similar trend for N balance was observed by Baniasadi et al. [9].
The ash content in the biochar increased with increasing temperature due to a concentration effect (Table 2). The Cl and S contents also increased, indicating that part of it was of inorganic origin [62,63,64]. The mass balance calculations for S showed that 27.8 to 41.2 wt.% of initial S was retained in the biochars for FCL compared to 61 wt.% for PCL. However, the amount of S retained in the biochars was lower compared to observations made by Baniasadi et al. [9].
3.3 Composition of gaseous products
Results of pyrolytic gas analysis from FCL and PCL feedstock are presented in Fig. 2a and b.
The combustible gaseous compounds identified for both feedstocks were predominantly carbon monoxide (CO), methane (CH4), hydrogen (H2), ethylene (C2H4), and ethane (C2H6). The combined volume fraction of combustible gases increased with temperature. This was slightly higher for FCL and was measured in the range from 43 to 73 vol.%, compared to 38 to 68 vol.% for PCL. An increase in the calorific value of pyrolysis gas, as presented in Table 3, could be directly linked to the increase of the volumetric fraction of combustible gases (CO, H2, CH4) at elevated temperatures, most likely associated with secondary tar reforming at higher pyrolysis temperature [65]. The HHV of the pyrolysis gas obtained from FCL and PCL at 500 and 600 °C was lower compared to results reported for slow pyrolysis of dried poultry litter by [9]. However, the calorific value of the evolved gases from both FCL and PCL at 700 °C was higher.
During pyrolysis, nitrogen is mainly released as NH3 [67] but also as nitrogen-containing organic and water-soluble compounds [9]. The mass balance calculations revealed that 75 to 90 wt.% of nitrogen and 40 to 70 wt.% of sulphur was released in the gaseous form. Moreover, the vaporisation of nitrogen and sulphur was more prominent at higher temperatures. If pyrolytic gases are not treated before being utilised in the downstream combustion unit [66], it could lead to higher NOx and SOxemissions.
3.4 Composition of liquid products
The liquid product from pyrolysis of FCL and PCL consisted of two phases, the aqueous phase which mainly contained water-soluble compounds and the organic phase (a.k.a. pyrolysis oil, tar). In this study, the composition of both phases was measured together. The water content in the liquid product was 70.8–80.4 vol.% from FCL and 73.5–78.2 vol.% from PCL (Table 3). Very high water content is a result of high moisture content in the feedstock and water vapour formed as a result of decomposition of organic matter during pyrolysis. GC-MS was used to measure the composition of the liquid product, but it was set not to detect the water molecule, which means the identified and quantified liquid compounds are given on a water free basis. Approx. 100 compounds can be detected in each chromatogram. NIST 11 MS library within MassHunter was used to identify organic compounds in the liquid product. Complete GC-MS data is given in supplementary material, Figs S-2 to S-7 and Tables S-2 to S-4; additionally, Table 5 provides results for pyrolysis at 500 °C.
The chemical nature of the organic compounds in Table 5 and Tables S-2 to S-4 depends on the pyrolysis temperature [68]. It is in accordance with the maturity of pyrolysis oil proposed by Elliot [16]. In this study, the organic fraction in the liquid product at 500 °C contained short-chain organic acids (i.e. propanoic and butanoic acid) and heterocyclic aromatics compounds such as pyrazines, pyridines, phenols, and their derivatives. Aromatic hydrocarbons, such as styrene, naphthalene, anthracene, and pyrene, appeared in notable quantities at 600 and 700 °C. According to Elliot’s [16] classification, polycyclic aromatic hydrocarbons belong to the secondary and tertiary group of pyrolysis oil compounds.
Although over 100 compounds have been identified in the liquid product, their amounts are relatively small. The organic phase obtained at the lowest pyrolysis temperature 500 °C contained propionic acid, Table 5, and most likely formic acid that due to the m/z setting of the mass spectrometer was not detected. Formic and propanoic acids could potentially be used for the production of environmentally friendly road de-icers [22]. Table 5 and Tables S-2 and S-3 in the supplementary material show that the liquid product contained notable quantities of valuable phenolic compounds. However, production of specific compounds from pyrolysis liquid product is seldom practical because of the complex and costly separation techniques in a larger scale application [22]. If the liquid product from pyrolysis is condense out, it would need to be disposed of.
3.5 Energy balance
The HHV of each pyrolysis product is summarised in Table 3. The HHVs of FCL and PCL biochars were higher than those reported by Baniasadi et al. [9]. This may be related to a lower ash content but higher fixed carbon in FCL and PCL compared to poultry litter used by Baniasadi et al [9]. The HHV of the liquid fraction from intermediate pyrolysis of FCL and PCL in the range from 10 to 18 MJ/kg was much lower compared to fast pyrolysis oil 26–29 MJ/kg [69] and 33 MJ/kg [15].
Figure 3 shows the proportion of potential combustion energy in each pyrolysis product, calculated based on the HHV presented in Table 3 and multiplied by their respective product yield (as measured). As expected by increasing the pyrolysis temperature, the amount of energy transferred to the gas phase increased while that in the biochar decreased.
A comparison between an estimated total energy required for pyrolysis of FCL/PCL against the potential combustion energy of pyrolysis gas was carried out. The total energy required to treat FCL and PCL, through pyrolysis (Qtotal), can be split into three consecutive processes occurring in the pyrolysis reactor: drying, heating to target temperature, and pyrolysis [70]. The calculations were carried out for 1 kg of FCL/PCL samples as an input material with moisture content as received (Table 2). The energy requirement for drying (Qdrying) was estimated by adding the heat required to increase the temperature of the FCL/PCL from ambient (15 °C) to 105 °C, plus the latent heat required to evaporate the water from the FCL and PCL. The heat capacity of dried FCL and PCL was assumed to be 1.4 kJ/kg °C [71]. The heat capacity of FCL and PCL including moisture was calculated using the rule of mixture and was 2.03 kJ/kg °C and 1.5 kJ/kg °C, respectively. The energy required to increase the temperature of the dried FCL/ PCL from 105 °C to 500/600/700 °C (Qtarget) was calculated using heat capacity of dry feedstock [70]. The energy required to decompose FCL/PCL during the pyrolysis reaction (Qpyro) was taken as for poultry litter 136 kJ/kg [9].
The results of these calculations, presented in Table 6, show that both FCL and PCL allow for energetically sustainable pyrolysis (Qavail/Qtotal = 4–7.6) to recover biochar for nutrient recycling, even when the process is carried out at 500 °C. The energy surplus is greater from the combustion of hot pyrolysis gas from PCL, since the PCL moisture content is lower compared to FCL. The above calculation did not include the heat required to heat up the reactor, nor does it account for heat losses. However, with an optimised design of process installation and operational procedure, these amounts could be kept to a minimum.
3.6 Potential application of chicken litter biochars
3.6.1 Nutrient availability focusing on phosphorus (P) speciation
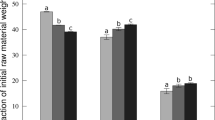
Phosphorus speciation in FCL, PCL, and derived biochar is presented in Fig. 4. In the current study, about 50 % and 60 % of total P was in a water-soluble form, referring to FCL and PCL, respectively (Fig. 4a). This agrees with previous findings [32, 59]. In pyrolysis biochars, only 3 to 9 % of the total P was water-soluble. Water-soluble P can leach into the ground/surface water [72], which may lead to eutrophication. Therefore, the transformation of phosphorus during pyrolysis into a non-water-soluble form is beneficial from an environmental perspective. The organic P contained in both FCL and PCL was about 60 % (Fig. 4b), which is in agreement with the literature [36]. As the organic matter partially decomposed during pyrolysis, the relative content of organically associated P decreased in FCL while the inorganic P contained in biochars was about 80 %. On the other hand, the organic P did not change or increase in PCL. In both FCL and PCL, 30 % of the total P was apatite P, whereas about 20 % was non-apatite inorganic P. Pyrolysis increased the apatite P in both feedstock biochars. Apatite P represented 40–47 % and 60–65 %, respectively, of total P in FCL- and PCL-derived biochars (Fig. 4c).
An increase in pyrolysis temperature from 500 to 700 °C had a moderate effect on phosphorus speciation. The feedstock composition seems to be more significant/important than process temperature for transformation of water-soluble and organic P into apatite P. The initial content of calcium in PCL was much higher compared to FCL (Table S-1 in supplementary information) (Ca/P molar ratio of 1.0 vs. 0.36, respectively); this resulted in an overall higher apatite formation in PCL biochars.
The concentration of other macronutrients in biochars is presented in Table 7. FCL and PCL biochars contain about 10 % of K, 6.5–9 % of P, 1.5–4.1 % Mg, 2.5–6 % Ca, 1.4–1.9 % of Na, and 2.2–3.2 % of S.
3.6.2 Innocuity, focusing on heavy metal content
The concentration of inorganic contaminants in FCL and PCL biochars was compared with the upper limits in the FPR legislation (Table 7). The Cr presented in Table 7 refers to the total Cr content instead of Cr (VI). Therefore, it is not possible to conclude whether biochars meet Cr (VI) criteria. The content of Ni in PCL biochar obtained at 700 °C slightly exceeds the legislation limit. Also, the content of Zn in FCL biochar produced at 600 and 700 °C exceeds the given limit. Thus, it is concluded that only biochars produced at 500 °C met the criteria set out in the FPR regulations, but biochars produced at higher temperatures did not meet the criteria.
3.7 Requirements for research for process optimisation and revenues
The energy balance presented in Section 3.5 shows that pyrolysis of FCL and PCL even at 500 °C produces gas (non-condensable gases and condensable organic fraction) that when combusted, it may provide sufficient heat to make the process energetically sustainable. However, further studies are required to provide evidence that flue gas from combustion of pyrolysis gas produced from feedstocks with high N content, such as chicken litter, can meet stringent criteria for emissions.
FCL/PCL biochar is a sterile, solid carbon, and nutrient-rich material (C content 50–58 wt.%, N content 2.0–4.5 wt. %, P content 2.9–4.0 %, K content 3.8–9.3 wt. %) that is easy to store and transport long distances, but its main disadvantage is that most of the nitrogen was lost in a gaseous form (at 500 °C about 75 wt.% and at 700 °C 90 wt.%). The content of water-soluble P in biochar was significantly reduced compared to the original FCL/PCL (from 50 to 3 % and 60 to 9 %); thus, pyrolysis turned FCL/PCL into biochar that when used as a soil amendment will reduce phosphorus losses to surface water. On the other hand, organic P was mineralised during pyrolysis, with increased formation of apatite P (FCL 40–47 % and PCL 60–65 % of total P), which is considered less plant available [73] compared to non-apatite inorganic P. However, a recent study showed that apatite P is partially available to plants, but the degree of its utilisation depends on the overall nutrient status and soil water distribution [35] suggesting that for some soils, the biochars may be a slow release source of P. Phosphorus availability to plants is an important factor for soil amendments/biochars, and results from this study suggest that temperature of 500 °C would be optimal out of all tested temperatures. A similar temperature had been suggested previously [31, 32]. Moreover, although heavy metals are concentrated in biochar, the content of Zn in biochar produced at 500 °C was below the upper limit given in the FPR.
To summarise, FCL/PCL properties enable energetically sustainable production of biochar containing recalcitrant carbon [74] for carbon sequestration with concentrated phosphorus and heavy metals. However, more studies should be carried out to show evidence that the heavy metals will not pose an environmental risk when used for soil amendment and to demonstrate if in fact pyrolysis can create a slow-release source of P.
4 Conclusion
Pyrolysis of fresh and pelletised chicken litter (FCL and PCL) was experimentally studied at 500, 600, and 700 °C. The product yield distribution was determined, and the properties of pyrolysis gas, liquid product, and biochar were examined. It can be concluded that (1) the biochar yield was lower while the liquid and gas yields were higher for FCL compared to PCL, mainly due to a higher initial moisture content; (2) the yield of biochar decreased with increasing pyrolysis temperatures resulting in higher gas yield; (3) pyrolysis concentrated macronutrients P, K, Ca, and Mg in biochars but also heavy metals. Transformation of water-soluble and organic P into inorganic P is occurring during pyrolysis, which is beneficial from an environmental perspective. However around 40 to 60 wt.% of total P in the biochar is apatite P, which means it is not directly available to plants in the short term. The content of apatite P depends primarily on the composition of the mineral matter in the feedstock, while a pyrolysis temperature in the range 500–700 °C had a negligible effect; (4) pyrolysis concentrated heavy metals, such as Zn and Ni, content of which increased in FCL/PCL biochar with increasing pyrolysis temperature. Only biochar produced at 500 °C met the eligibility criteria set out in the FPR regulation; (5) mass and energy balance showed that pyrolysis of FCL/PCL even at 500 °C produces hot gases/vapours whose combustion may render the process energetically sustainable; (6) pelletisation of chicken litter prior to pyrolysis (PCL) led to much higher surplus of energy from combustion of hot pyrolysis gas, compared to FCL. However, FCL also provided sufficient energy to allow for an energetically sustainable pyrolysis process; thus, pelletisation of chicken litter is not needed, unless application of pelletised biochar is preferred.
Pyrolysis is a promising and disruptive technology for low quality feedstock such as chicken litter and has shown the possibility to recover energy through direct combustion of pyrolytic gases. It also may reduce losses of phosphorus to ground waters and eutrophication when biochar is used as a soil amendment/fertiliser instead of chicken litter. However, heavy metals may restrict the utilisation of biochars in agriculture; therefore, more research is required to identify and manage its sources, e.g. the dietary practices need to be changed, to reduce Zn in the chicken feed.
Data availability
Not applicable
References
Shahbandeh M (2022) Poultry industry in the United Kingdom- statistics & facts. Available from: https://www.statista.com/topics/6102/poultry-in-the-united-kingdom/#topicHeader__wrapper
Cantrell KB et al (2008) Livestock waste-to-bioenergy generation opportunities. Bioresour Technol 99(17):7941–7953
Rout PR et al (2023) Sustainable valorisation of animal manures via thermochemical conversion technologies: an inclusive review on recent trends. Waste Biomass Valori 14(2):553–582
Gerber PJ et al (2013) Tackling climate change through livestock: a global assessment of emissions and mitigation opportunities. Food and Agriculture Organization of the United Nations (FAO), Rome
Kim WK, Patterson PH (2003) Effect of minerals on activity of microbial uricase to reduce ammonia volatilization in poultry manure. Poult Sci 82(2):223–231
Lynch D et al (2013) Utilisation of poultry litter as an energy feedstock. Biomass Bioenergy 49:197–204
Huang Y et al (2015) Biochar and renewable energy generation from poultry litter waste: a technical and economic analysis based on computational simulations. Appl Energy 160:656–663
Basu P (2010) Biomass gasification and pyrolysis: practical design and theory. Elsevier Inc., p 365
Baniasadi M et al (2016) Waste to energy valorization of poultry litter by slow pyrolysis. Renew Energy 90:458–468
Simbolon LM et al (2019) Investigation of chicken litter conversion into useful energy resources by using low temperature pyrolysis. Energy Procedia 161:47–56
Kim S-S, Agblevor FA, Lim J (2009) Fast pyrolysis of chicken litter and turkey litter in a fluidized bed reactor. J Ind Eng Chem 15(2):247–252
Simbolon LM, Pandey DS, Tsekos C, de Jong W, Tassou SA (2019) Investigation of poultry litter conversion into useful energy resources using fast pyrolysis
Santos Dalólio F et al (2017) Poultry litter as biomass energy: a review and future perspectives. Renew Sustain Energy Rev 76:941–949
Cantrell KB et al (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428
Pandey DS et al (2019) Fast pyrolysis of poultry litter in a bubbling fluidised bed reactor: energy and nutrient recovery. Sustainability 11(9):2533
Elliott DC (1988) Relation of reaction time and temperature to chemical composition of pyrolysis oils. In: pyrolysis oils from biomass. American Chemical Society, pp 55–65
Gollakota ARK et al (2016) A review on the upgradation techniques of pyrolysis oil. Renew Sustain Energy Rev 58:1543–1568
Horvat A et al (2019) Tar yield and composition from poultry litter gasification in a fluidised bed reactor: effects of equivalence ratio, temperature and limestone addition. RSC Adv 9(23):13283–13296
Arnold S et al (2017) Biotechnological perspectives of pyrolysis oil for a bio-based economy. Trends Biotechnol 35(10):925–936
Moita R, Lemos PC (2012) Biopolymers production from mixed cultures and pyrolysis by-products. J Biotechnol 157(4):578–583
Oasmaa A, Kytö M, Sipilä K (2001) Pyrolysis oil combustion tests in an industrial boiler, in progress in thermochemical biomass conversion. 1468-1481
Czernik S, Bridgwater AV (2004) Overview of applications of biomass fast pyrolysis oil. Energy Fuels 18(2):590–598
Tomasi Morgano M et al (2018) Screw pyrolysis technology for sewage sludge treatment. Waste Manag 73:487–495
Morgano MT et al (2018) Intermediate pyrolysis of agricultural waste: a decentral approach towards circular economy. Chem Eng Trans
Chan KY et al (2008) Using poultry litter biochars as soil amendments. Soil Res 46:437–444
Sikder S, Joardar JC (2019) Biochar production from poultry litter as management approach and effects on plant growth. IJROWA 8(1):47–58
Solaiman ZM et al (2020) Poultry litter biochar increases mycorrhizal colonisation, soil fertility and cucumber yield in a fertigation system on sandy soil. Agriculture 10(10):480
Ofori P et al (2021) Combined application of poultry litter biochar and NPK fertilizer improves cabbage yield and soil chemical properties. Open Agric 6(1):356–368
Hadroug S et al (2019) Pyrolysis process as a sustainable management option of poultry manure: characterization of the derived biochars and assessment of their nutrient release capacities. Water 11(11):2271
Sun K et al (2018) Speciation of phosphorus in plant- and manure-derived biochars and its dissolution under various aqueous conditions. Sci Total Environ 634:1300–1307
Sarvi M et al (2021) Bioavailability of phosphorus in granulated and pyrolyzed broiler manure. Environ Technol Innov 23:101584
Bergfeldt B et al (2018) Recovery of phosphorus and other nutrients during pyrolysis of chicken manure. Agriculture 8(12):187
Péret B et al (2011) Root developmental adaptation to phosphate starvation: better safe than sorry. Trends Plant Sci 16(8):442–450
Santner J et al (2012) High-resolution chemical imaging of labile phosphorus in the rhizosphere of Brassica napus L. cultivars. EEB 77:219–226
Wolff J et al (2020) Bioavailability and accessibility of subsoil allocated 33P-labelled hydroxyapatite to wheat under different moisture supply. Sci Rep 10(1):17140
Barnett GM (1994) Phosphorus forms in animal manure. Bioresour Technol 49(2):139–147
Olukosi OA, van Kuijk S, Han Y (2018) Copper and zinc sources and levels of zinc inclusion influence growth performance, tissue trace mineral content, and carcass yield of broiler chickens. Poult Sci 97(11):3891–3898
Sistani KR, Novak JM (2005) Trace metal accumulation, movement, and remediation in soils receiving animal manure. In: Trace elements in the environment. CRC Press, pp 707–724
Luyckx L, de Leeuw GHJ, Van Caneghem J (2020) Characterization of poultry litter ash in view of its valorization. Waste Biomass Valori 11(10):5333–5348
Taupe NC et al (2016) Updraft gasification of poultry litter at farm-scale – a case study. Waste Manag 50:324–333
Nortcliff S and Gregory PJ (2013) The historical development of studies on soil–plant interactions, in Soil Conditions and Plant Growth. 1-21
Maroušek J, Strunecký O, Stehel V (2019) Biochar farming: defining economically perspective applications. Clean Technol Environ Policy 21(7):1389–1395
Samoraj M et al (2022) Biochar in environmental friendly fertilizers - prospects of development products and technologies. Chemosphere 296:133975
EC E.C (2019) EU Fertilising Products Regulation 2019/1009, in 2019/1009, T.E.P.A.T. COUNCIL, Editor. Official Journal of the European Union
Usero J et al (1998) Comparative study of three sequential extraction procedures for metals in marine sediments. Environ Int 24(4):487–496
Ure AM et al (1993) Speciation of heavy metals in soils and sediments: an account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the Commission of the European Communities. Int J Environ Anal Chem 51:135–155
Wang A et al (2021) Speciation and environmental risk of heavy metals in biochars produced by pyrolysis of chicken manure and water-washed swine manure. Sci Rep 11(1):11994
Yang K et al (2023) Effects of physical aging processes on the bioavailability of heavy metals in contaminated site soil amended with chicken manure and wheat straw biochars. Environ Pollut 324:121414
Jerzak W, Reinmöller M, Magdziarz A (2022) Estimation of the heat required for intermediate pyrolysis of biomass. Environ Pollut 24(10):3061–3075
Nardis BO et al (2022) Production of engineered-biochar under different pyrolysis conditions for phosphorus removal from aqueous solution. Sci Total Environ 816:151559
Slezak R et al (2023) An extensive review and comparison of modern biomass reactors torrefaction vs. biomass pyrolizers-Part 2. Energies 16(5):2212
BS, Solid Biofuels (2009) Determination of moisture content. Oven dry method. Moisture in general analysis sample, EN
BS, Solid Biofuels (2009b) Determination of ash content. EN
BS, Solid Biofuels (2009c) Determination of the content of volatile matter. EN
BS, Solid Recovered Fuels ( 2011) Methods for the Determination of Sulphur (S), Chlorine (Cl), Fluorine (F) and Bromine (Br) Content, EN
Horvat A et al (2016) Detailed measurement uncertainty analysis of solid-phase adsorption-total gas chromatography (GC)-detectable tar from biomass gasification. Energy Fuels 30(3):2187–2197
García-Albacete M, Martín A, Cartagena MC (2012) Fractionation of phosphorus biowastes: characterisation and environmental risk. Waste Manag 32(6):1061–1068
Pardo P, López-Sánchez JF, Rauret G (2003) Relationships between phosphorus fractionation and major components in sediments using the SMT harmonised extraction procedure. Anal Bioanal Chem 376(2):248–254
Ghanim BM, Kwapinski W, Leahy JJ (2018) Speciation of nutrients in hydrochar produced from hydrothermal carbonization of poultry litter under different treatment conditions. ACS Sustain Chem Eng 6(9):11265–11272
Demirbas A (2004) Effect of initial moisture content on the yields of oily products from pyrolysis of biomass. JAAP 71(2):803–815
Xiong S et al (2013) Effect of moisture content on the characterization of products from the pyrolysis of sewage sludge. JAAP 104:632–639
Middleton SP, Patrick JW, Walker A (1997) The release of coal nitrogen and sulfur on pyrolysis and partial gasification in a fluidized bed. Fuel 76(13):1195–1200
Knudsen JN et al (2005) Secondary capture of chlorine and sulfur during thermal conversion of biomass. Energy Fuels 19(2):606–617
Kaufman Rechulski MD et al (2014) Sulfur containing organic compounds in the raw producer gas of wood and grass gasification. Fuel 128:330–339
Morf P, Hasler P, Nussbaumer T (2002) Mechanisms and kinetics of homogeneous secondary reactions of tar from continuous pyrolysis of wood chips. Fuel 81(7):843–853
Czajczyńska D, Krzyżyńska R, Jouhara H (2022) Hydrogen sulfide removal from waste tyre pyrolysis gas by inorganics. Int J Hydrog
Clark SC et al (2017) Effluent gas flux characterization during pyrolysis of chicken manure. GC11A-G0723
Biswas B et al (2018) Pyrolysis behavior of rice straw under carbon dioxide for production of bio-oil. RE 129:686–694
Agblevor FA et al (2010) Biocrude oils from the fast pyrolysis of poultry litter and hardwood. Waste Manag 30(2):298–307
Kim Y, Parker W (2008) A technical and economic evaluation of the pyrolysis of sewage sludge for the production of bio-oil. Bioresour Technol 99(5):1409–1416
Ahn HK et al (2009) Determination of thermal properties of composting bulking materials. Bioresour Technol 100(17):3974–3981
McGechan MB (2010) Modelling water pollution by leached soluble phosphorus, part 2: simulation of effects of manure management. Biosyst Eng 106(3):250–259
Kratz S, Vogel C, Adam C (2019) Agronomic performance of P recycling fertilizers and methods to predict it: a review. Nutr Cycl Agroecosystems 115(1):1–39
Song W, Guo M (2012) Quality variations of poultry litter biochar generated at different pyrolysis temperatures. JAAP 94:138–145
Funding
Open Access funding provided by the IReL Consortium LMS acknowledges the postgraduate research scholarship received from the Government of Indonesia through the Ministry of Education and Culture and Politeknik Negeri Bandung. Also, acknowledged is support provided by the Centre for Sustainable Energy Use in Food Chains (CSEF) through RCUK Grant No. EPSRC Grant ref EP/K011820/1.
Author information
Authors and Affiliations
Contributions
Conceptualization: DSP, MK. Methodology: DSP, AH, MK. Validation: LMS. Investigation: LMS. Resources: SAT, JJL. Data curation: LMS, AH, MK. Writing original draft: LMS, DSP, AH, MK. Writing review and editing: DSP, AH, MK, JJL. Visualisation: LMS, AH. Supervision: DSP, AH, MK, SAT. Funding acquisition: LMS, SAT
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simbolon, L.M., Pandey, D.S., Horvat, A. et al. Influence of the pyrolysis temperature on fresh and pelletised chicken litter with focus on sustainable production and utilisation of biochar. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04787-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04787-5