Abstract
Standardization of the cultivation conditions is crucial for producing fungal biomass with effective whole-cell catalytic activity. Aspergillus flavus mutant was optimized for biomass transesterification and hydrolysis of waste frying oil (WFO) as a substrate. The glucose-polypeptone medium was selected among other culture media as the primary medium for the optimization of submerged cultivation. Biomass lipolytic activities and growth of A. flavus were higher under shaking conditions compared to static conditions, and 200 rpm was optimum. Olive oil was superior to soybean, rapeseed, and waste frying oils as biomass lipase and transesterification activities inducer. Replacing glucose with sugar cane molasses enhanced the growth but inhibited the biomass lipolytic capabilities. Urea improved the biomass lipolytic capabilities as an N-source feedstock compared to corn-steep liquor and whey. Plackett Burman screening of 9 factors showed that KH2PO4 (0.1–0.9 %, w/v), urea (0.1–1.3 %, w/v), and glucose (0.5–3.5 %, w/v) concentrations were significant factors with the highest main effect estimates 38.45, −19.04, and 11.82, respectively. These factors were selected for response surface methodology (RSM) optimization using central composite design (CCD). CCD models for growth, biomass lipase activity, and transesterification capability were significant, with R2 values of 0.93, 0.91, and 0.95, respectively. The optimum concentrations for growth and biomass transesterification capability were glucose (4.5 %, w/v), urea (0.7 %, w/v), and KH2PO4 (0.9 %, w/v), and those for lipase activity of the biomass were glucose (4.4 %, w/v), urea (0.7 %, w/v), and KH2PO4 (2.1 %, w/v).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fungal whole-cell biocatalysts have been reported as a potent alternative for pure enzymes that require multiple purification steps. Whole-cell biocatalysts provide a protective environment to the cell wall integrated enzymes and cost reduction of the catalysis process[1]. Several factors may play essential roles in the improvement of the whole-cell catalytic activity that relies on the investigated species, mainly: (a) the diversity and number of genes of the catalytic protein of interest, (b) the cell wall synthesis mechanism and the mechanism(s) of protein integration into the cell wall, and (c) the presence or absence of mucilage cell wall layer or biofilm that might act as a physical barrier [2,3,4]. However, optimizing the cultivation conditions is critical for producing fungal biomass with potent whole-cell catalytic activity.
Various fungi have been investigated for enzymatic activities and industrial applications [5, 6]. Aspergilli are more than 340 species; only a few are used for food fermentation, organic acids, and bioactive compound production. Aspergilli of industrial importance are mainly distributed in two groups, Nigri and Flavi [7]. A study of Aspergillus flavus genomics suggested that further investigations of A. flavus may lead to the discovery of new enzymes and pharmaceutical compounds [8]. A. flavus isolates cultivated under optimized conditions were found to be remarkable candidates for industrial enzyme production [9]. Whole-cell Aspergilli have been studied to replace pure enzymes such as cellulases [10] and lipases [11].
Classical optimization, one factor at a time (OFAT), is employed by investigating one factor or variable while all other variables remain constant. OFAT is considered an easy convenience; hence, it has been the most preferred choice among the researchers for designing the medium composition and used in the initial stages of diverse strategies. The main disadvantages of OFAT optimization are the inability to predict the interaction among investigated variables and the vast number of experimental runs required that are not applicable on a large scale [12]. On the other hand, statistical optimization provides attractive screening and optimization designs where the number of runs necessary is limited, and the interaction of the investigated factors might be evaluated. Hence, a single set of optimum conditions could be identified, and several sweet spots may also be located. Statistical optimization designs require a prior understanding of the logic ranges of the investigated variables and responses [13].
In this research, OFAT, screening design, and optimization design were employed to maximize the biomass catalytic activity of A. flavus for waste frying oil hydrolysis and transesterification, as well as the biomass produced. Understanding the impact of the investigated factors and their interaction with the researched responses was also pursued.
2 Materials and methods
2.1 Fungal strain and inoculum preparation
In a previous study, A. flavus NDA04a (MK811208) mutant D was isolated and mutated [14]. The mutant was preserved on potato dextrose agar (PDA) slants supplemented with olive oil, 1%. The inoculum was prepared by subculturing the mutant on PDA plates incubated at 28 °C for three days. Agar disks (5 mm diameter) were used as inoculum.
2.2 Culture media and feedstock screening
Different culture media (Table 1) were tested as the primary cultivation medium. Olive oil 3 %, w/v, was supplemented for all tested media as an inducer, and the final pH of the tested media was adjusted to 5.5. Erlenmeyer flasks (100 mL capacity) containing 50 mL cultures were inoculated using a 5mm disk of PDA fresh culture and incubated at 28°C and 150 rpm for three days. Harvested biomass was collected by filtration using Whatman filter paper no. 1, followed by lyophilization using a freeze dryer (Martin Christ, alpha LSC basic; Germany).
Glucose-polypeptone medium (A) supplemented with olive oil 30 mL L−1 was used as the primary medium for the cultivation of A. flavus NDA04a and assessment of its biomass transesterification capabilities. Sugar cane molasses was tested to replace glucose as a C source, while corn steep liquor (CSL), urea, and whey were tested as N sources with and without Molasses. Inoculated cultures were incubated in a shaking incubator at 28 °C and 150 rpm for three days.
2.3 Screening for transesterification activity stimulants
Glucose-polypeptone media were prepared using different activity stimulants (3%, w/v), including olive, soybean, rapeseed (Carl-Roth, Karlsruhe, Germany), and waste frying oils. Inoculated cultures were incubated in an orbital shaker at 150 rpm and 28 °C for three days. Harvested biomass was processed, and the activities were determined.
2.4 Investigation of agitation rate
Triplicates of glucose-polypeptone 100 mL inoculated cultures in 250 mL Erlenmeyer flasks were incubated in shaking incubators with the same orbital diameter at 28 °C and different agitation rates (0, 100, 200, and 300 rpm) for three days. Biomass was harvested and processed, as previously mentioned, to measure biomass lipolytic and transesterification activities.
2.5 Statistical screening
Plackett Burman design was used for screening experiments of 9 factors (Table 2) in triplicates. The screening experiments were carried out in 100 mL conical flasks containing 50 mL culture medium. A three-day-old culture of A. flavus NDA04a was used as the source of the inocula. Flasks were incubated in a shaking incubator at 200 rpm. Biomass was processed, and the lipolytic and transesterification activities of the biomass were determined.
2.6 Response surface methodology (RSM)
Glucose, urea, and KH2PO4 concentrations were optimized using a central composite design (CCD). The investigated levels of each factor are presented in Table 3. Culture media were prepared using 0.9 g L−1 MgSO4 and 2 mL L−1 olive oil with different levels of investigated factors and their relative interactions as the design suggested in triplicates. Cultures were inoculated with three disks 5 mm in diameter and were incubated at 28 °C and 200 rpm for four days. The processed biomass was used to assess the transesterification activity of the biomass.
2.7 Lipase assay
Lyophilized fractured biomass (0.5 g) was inoculated to an emulsion of 5.5 g WFO and 30 mL of 1M Tris buffer, pH 7.5. The reaction was carried out in an orbital shaker at 35 °C and 200 rpm for 2 h. The reaction mixture was centrifuged for 10 min at 5000 rpm. Supernatant (1 g), two drops of phenolphthalein color indicator, and 25 mL diethyl ether and ethanol (1:1) solvent mixture were titrated against freshly prepared 0.1 N NaOH in 100 mL Erlenmeyer flask. Lipase activity (as the amount of enzyme required to produce 1 μmol free fatty acid per min.) was determined in unit per gram cell weight (U/g) [15].
2.8 Transesterification reaction
Fractured lyophilized biomass, 0.5 g, was used as the biocatalyst for an emulsion of 5 g WFO and 0.75 mL of 1M phosphate buffer pH 7.5 in a 50 mL Erlenmeyer flask. The reaction was carried out at 35 °C and 250 rpm for 72 h, while methanol doses were added at 0, 24, and 48 h to a final concentration of 3 M methanol. The reaction mixture was transferred to a 15 mL centrifuge tube and spun at 10000 rpm for 5 min.
Fatty acid methyl ester (FAME) was detected in the supernatant by thin-layer chromatography (TLC) with silica gel 60 F254 (E. Merck, Mumbai, India) using a solvent system of hexane/diethyl ether/acetic acid. Spots were stained in an iodine chamber and were investigated by Just TLC software (Sweday, Lund, Sweden).
2.9 FAME analysis
Agilent Technologies 6890N gas chromatography (GC) provided a flame ionization detector, and a capillary column (HP-5 5% phenyl methyl siloxane, 30 m by 320 μm by 0.25 mm) was used to quantify the FAMEs content. Peaks determination was carried out by comparing the retention time of FAMEs of the sample (100 mg) and a known concentration of FAME standard mixture C8–C24 (Sigma-Aldrich Chemical Co. St. Louis, MO, USA), each dissolved in 1 mL hexane. One μL sample was injected into the GC, where the oven was adjusted at 210 °C, isothermally for 15 min, and helium was used as the carrier gas. The peak area was used to calculate the concentration of each FAME type, and the sum of the concentration was referred to as FAME concentration (%, w/w). The transesterification activity was expressed using FAME concentration (%, w/w).
2.10 Statistical analysis
The data obtained were subjected to statistical analysis using IBM SPSS Statistics (version 16. IBM, Chicago, USA). Statistical optimization was designed and analyzed using JMP statistical software (version 8. SAS Institute Inc., Cary, NC).
3 Results and discussion
3.1 One factor at time investigations
3.1.1 Different culture media investigation
Different culture media were used to cultivate A. flavus NDA04a. The produced biomass was then lyophilized and used as the catalyst for waste frying oil hydrolysis and transesterification reactions. One-way analysis of variances (ANOVA) showed that culture media composition significantly influenced the biomass’s growth, lipase, and transesterification activities. A. flavus NDA04a showed a final lyophilized biomass range from 11.58 to 18.30 g/L (Table 4). The maximum growth was observed when A. flavus NDA04a was cultivated on culture media A, followed by culture media B with a significant difference. On the other hand, the minimum growth was observed when A. flavus NDA04a was grown on culture media C and D with no significant difference. Although culture media A and B showed a significant difference in the final lyophilized biomass concentration, biomass lipase, and transesterification activities were insignificantly different. A. flavus NDA04a investigated biomass catalytic capabilities were significantly higher for biomass cultivated on culture media A and B than other culture media. Although olive oil as a lipase stimulant had the same concentration in all investigated culture media, the lipases and transesterification activities of biomass cultivated in culture media C and D were significantly low. These reduced activities and final biomass concentration may result from carbon or nitrogen sources and ratio variations. Culture media A and B had almost the same composition, which might explain the insignificant differences in biomass activities. Although glucose as an additional carbon source in culture media A did not increase the biomass catalytic activities, glucose may be the reason for the significant increase in the final biomass concentration. As the biomass lipid modification activities are the responses of interest, the final biomass concentration is also essential; therefore, culture medium A was found superior to B and was selected for further investigations.
3.1.2 Effect of different agitation rate
Biomass morphology affects Aspergillus sp. catalytic activities [16]. Hence, the shaking rate was investigated as one of the main factors determining culture morphology. Results showed that the shaking rate significantly affects the growth, biomass lipase activity, and transesterification catalytic capabilities. A. flavus NDA04a was used to inoculate sterile Erlenmeyer flasks containing media A. Cultures were incubated for 72 h at 28 °C and different agitation rates. Freeze-dried biomass was later used as the catalyst for waste frying oil hydrolysis and transesterification reactions. Table 5 shows that static culture had significantly lower growth and catalytic activities than shaking cultures. An increase in shaking speed from 100 rpm to 200 rpm led to a significant increase in biomass (56.85%), lipase activity (10.24 %), and FAME production (2.61 %). Morphology was a mycelial mat covered with aerial mycelia in static conditions. Pellets in shaking cultures were smaller in 200 and 300 rpm cultures compared to the relatively large pellets in 100 rpm cultures. The results show that pellets exhibit higher lipase and transesterification catalytic activities than mycelial mats. The pellet size may play a role in the activity that might be related to the exposed surface area to the oil during the hydrolysis and transesterification reactions. [17].
3.1.3 Selection of activity inducer
Triglycerides in the culture media of fungi serve as a source of fatty acids and energy. To utilize oils, a fungus must produce extracellular or cell wall-integrated lipases, so oils stimulate lipase production [18]. Therefore, different oils were tested in this experiment to increase the lipase catalytic activities of A. flavus NDA04a and to assess their influence on growth. Culture media A was supplemented with 3 % (w/v) of olive, soybean, rapeseed, and waste frying oil separately. Inoculated flasks were incubated at 28°C and 200 rpm for 72 h. Collected biomass was freeze-dried and used as the catalyst for waste frying oil hydrolysis and transesterification reactions. Data (Table 6) show that the investigated oils exert no significant effect on growth. No significant difference was detected between the means of the final biomass concentration. However, the lipase activities and transesterification capabilities were significantly affected by different oils. Olive oil stimulated the highest catalytic activities, which might be due to an increase in the content of lipases on the surface of the hyphae. These results agree with other findings that suggest the high oleic acid content of olive oil may be the inducer of the high lipolytic activities in the presence of olive oil [19, 20]. The effects of waste frying oil and rapeseed oil were almost identical, possibly due to the composition of the waste frying oil, which contains a high proportion of rapeseed oil. Soybean oil cultures showed the lowest catalytic activities; even so, several pieces of literature hosted soybean oil as an inducer of lipase production [21, 22].
3.1.4 Replacing carbon and nitrogen sources
Culture medium A supplemented with olive oil 3% w/v was used as the primary medium for cultivation. Sugar cane molasses were tested to replace glucose as a C source. Corn steep liquor (CSL), urea, and whey were tested as replacements for polypeptone with and in the presence and absence of Molasses. Inoculated cultures were incubated in a shaking incubator at 28 °C and 200 rpm for three days. Collected biomass was freeze-dried, and weight, lipase activity, and transesterification activity were measured and recorded (Table 7).
Molasses increased the final biomass concentration remarkably (28 gL−1). However, lipase and transesterification activities were inhibited entirely. The reason for the loss of the biomass catalytic activities might be sugars in molasses which provide a more reliable metabolic pathway and/or the presence of inhibitory substances in molasses. No growth was observed in molasses and whey mixture cultures, which suggests that the accumulation of inhibitory substances or osmolarity prevented the growth of A. flavus NDA04a [23].
One-way ANOVA revealed that replacing nitrogen sources in glucose cultures significantly affected the growth, biomass lipase, and transesterification capabilities of A. flavus NDA04a. The maximum growth was observed in cultures where polypeptone and CSL were the nitrogen sources, 18.45 and 19.29 g/L, respectively. Biomass obtained from CSL and whey showed a remarkable reduction in lipolytic and transesterification catalytic abilities, suggesting that they harbor some inhibitory substances or provide alternative metabolic pathways. Although cultures containing urea and glucose showed significantly less biomass production (14.35 g/L), the catalytic capabilities of the biomass were significantly high. Unlike urea, other feedstock were complex and might be a source of carbon, microelements, or growth promotors. Urea might push the strain to utilize the triglycerides, producing more cell wall integrated lipolytic proteins.
3.2 Statistical screening using Plackett Burman design
Nine factors were investigated using the Plackett Burmann design (Table 2). Transesterification catalytic capability in terms of FAME conversion percentage, lipase activity, and growth in terms of final biomass concentration was observed as responses. The design matrix selected for screening significant factors is shown in Table 8. The model adequacy was calculated. Influences of the investigated factors and statistical significance were filtered via main effect estimates, Student’s t-test, and P values for analysis of variance (Table 9). Factors with confidence greater than 90% (Prob. > │t│ 0.1) were considered to affect the response significantly. The lowest P values indicate the most significant factors in the investigated response.
The concentration of KH2PO4 (1–9 g L−1) was the factor with the highest positive main effect on growth, biomass lipase, and transesterification capabilities with a high significance (P value = 0.0001). In addition to the role of KH2PO4 as a source of phosphate and potassium required for growth and lipolytic activities [24], it might be essential in buffering the culture media [25, 26]. As the used culture media contain no buffer system, the buffering effect of KH2PO4 might be essential for the growth and biomass activities of A. flavus NDA04a, considering that growth and lipase production might be accompanied by a change in the final pH of the culture medium [27, 28]. The influence of the initial pH value might support the essential buffering role of KH2PO4, as it was found that initial pH (5–9) significantly affected the transesterification capability of A. flavus NDA04a biomass negatively and the growth positively.
As a cheap nitrogen source for microbial cultivation, urea was selected as a sole and additional N source for enhancing lipase production. In several studies, urea was optimal for extracellular and whole-cell lipase production [29,30,31]. However, urea concentration (1–13 g L−1) significantly negatively influenced A. flavus NDA04a biomass lipase and transesterification capabilities. Still, the urea concentration in the investigated range did not significantly affect the growth. The ability of A. flavus NDA04a to produce cell wall-integrated lipolytic enzymes was reduced at high urea concentrations. This might be because urea at high concentrations might act as a C-source [32], the impact of the C/N ratio, or the toxic effect of urea or ammonia produced from urea hydrolysis [33].
Glucose concentration positively influences the growth of A. flavus NDA04a in the investigated range, which its role as a C-source might explain. However, some literature suggests that glucose inhibits the lipolytic activity of some fungi [34,35,36]. Glucose was a significant factor that had a remarkably positive impact on the lipase and transesterification activities of A. flavus NDA04a, which agrees with the findings in several research articles [37,38,39].
Magnesium sulfate concentration in the range of 0.1 to 0.9 g L−1 was significant, positively impacting the growth, biomass lipase, and transesterification capabilities of A. flavus NDA04a. Sulfur is an essential macronutrient for all living organisms that play critical structural and metabolic roles [40] which might be a reason for these findings. Di-cations are substantial for fungal growth and as cofactors for several enzymes. Magnesium has been reported to enhance lipase production by fungi alone [41] or synergistically with other di-cations such as calcium [42], which could explain the positive effect of MgSO4 on the biomass lipase activity and transesterification capability.
Inoculum characteristics were described as a substantial variable affecting the growth of fungi and enzyme production [43]. Inoculum size (1–3, 5 mm disks) significantly influenced the investigated responses. The same goes for the incubation period, which plays a critical role in catalytic proteins’ biosynthesis and autolysis rate in Aspergillus sp. [44].
The temperature range of 25–30 °C had no significant effect on the biomass lipase activity and transesterification capability. Still, it showed a highly significant impact on the growth of A. flavus NDA04a. On the other hand, olive oil concentration (10–30 g L−1) had an insignificant influence on the tested criteria of A. flavus NDA04a.
3.3 Statistical optimization using response surface methodology (RSM)
According to PB design, KH2PO4 concentration, urea concentration, and glucose concentration had the highest significant main effects on biomass transesterification capability and lipase activity and significantly affected the growth; therefore, they were selected for further optimization. Other factors were fixed, where positive values were chosen for significant factors and central values for insignificant ones based on the biomass transesterification capability results.
To determine the optimum levels of the selected significant factors for A. flavus NDA04a biomass transesterification capability. For this purpose, the response surface methodology (RSM) was adopted, using a central composite design (CCD). The significant variables employed are as shown in Table 3. The design matrix and the corresponding results of RSM experiments to determine the effects of the three investigated factors are shown in Table 10.
The acceptability of the models was verified via the results of the analysis of variance, ANOVA (Table 11). Transesterification capability in terms of FAME (%), lipase activity (U/g), and growth in terms of freeze-dried biomass models were significant, with P values below 0.0001, and the lack of fit of the three models shows P values higher than 0.05. Blotting the actual values obtained from the experiments against the predicted values deducted by the model (Fig. 1) supports the findings of the adequacy of the models, where R2 values were 0.95, 0.91, and 0.92 for FAME (%), lipase activity, and growth, respectively.
The prediction formulas were simplified to second-order polynomial equations. The responses, FAME (Y1), lipase (Y2), and growth (Y3), can be expressed in terms of the following regression equations:
The transesterification capability model terms X6, X7, X52, X5X6, and X6X7 were highly significant with P values less than 0.0001, while X5X7 was significant with a very low P value (0.0489). The lipase activity model terms X5, X6, X7, X52, and X5X6 had P values less than 0.0001, X62 and X6X7 were also significant with P values of 0.0021 and 0.0023, respectively. On the other hand, only X5 and X6 were significant terms for the growth model with P values less than 0.0001.
The significant positive effect of X5X6 (glucose and urea concentrations interaction) on the transesterification and the lipase activities might result from the impact of the C/N ratio, which was reported for other Aspergilli and closely related members of Ascomycetes [45, 46]. Another interaction (X6X7, urea, and KH2PO4 concentrations) was also found significant for the biomass transesterification and lipase activities which might be understood in the light of the buffering capacity of KH2PO4 along with the tendency of urease activity that alters the culture medium pH. On the other hand, the interaction X6X7 (urea and KH2PO4 concentrations) had no significant influence on any of the tested responses.
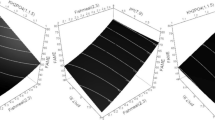
3D-surface plots were constructed to determine the optimum conditions, where the response was plotted on the Z-axis against the two of the investigated factors were plotted on the X and Y axes. In contrast, the third factor was set to the optimal value.
As shown in Fig. 2, linear increase in biomass transesterification capability was observed when the urea concentration was increased. A linear decrease in biomass transesterification capability was observed when the KH2PO4 concentration increased. The decline in the transesterification capability was associated with the increase in glucose concentration up to 38.1 g L−1. The model suggests the optimum cultivation conditions for A. flavus NDA04a biomass transesterification capability within the experimental space to be X5 = 4.5, X6 = 0.7, and X7 = 0.9 %, w/v.
Urea concentration in the investigated range shows a linear correlation to biomass lipase activity (Fig. 3), and the maximum lipase activity of the biomass was achieved at a high urea concentration. An increase in the lipase activity was associated with the rise in the glucose concentration up to 4.4%, w/v. A decline in the lipolytic activity was observed with a further increase in the glucose concentration. KH2PO4 concentration directly correlated with biomass lipase activity of A. flavus NDA04a. The model suggests the optimum cultivation conditions for A. flavus NDA04a biomass lipase activity within the experimental space to be X5 = 4.4, X6 = 0.7, and X7 = 2.1 %, w/v.
The growth (Fig. 4) shows a linear correlation with glucose and urea concentrations, where the increase in the concentration of urea and/or glucose leads to an increase in the biomass produced. KH2PO4 concentration in the investigated range had a highly insignificant influence on the growth of A. flavus NDA04a (P value = 0.7707); however, low concentrations of KH2PO4 were associated with relatively high biomass production. The model suggests the optimum cultivation conditions for A. flavus NDA04a growth within the experimental space to be X5 = 4.5, X6 = 0.7, and X7 = 0.9 %, w/v.
Determining optimum conditions for biomass transesterification capabilities and lipase activity should always consider the produced biomass as an essential factor for selecting a sweet spot where maximum activity per gram of biomass is achieved along with high biomass production. The optimum conditions for the biomass transesterification capability are identical to those required for the highest growth. For biomass lipase, the optimum conditions vary from those for biomass production in glucose and KH2PO4 concentrations. Increasing the glucose concentration from 4.4 to 4.5 %, w/v increases the growth without affecting the lipase activity of the produced biomass. Conversely, any reduction in the concentration of KH2PO4 has an apparent adverse effect on lipase activity but has an insignificant influence on the produced biomass.
Regarding biomass production, biomass lipase activity’s sweet spot would be X5 = 4.5, X6 = 0.7, and X7 = 2.1 %, w/v. Although the correlation between biomass lipase activity and transesterification capability is observable, the optimum conditions are different. This difference might result from the cell wall integrated lipases variation as some lipases have limited or no transesterification capability while having a high hydrolysis activity [47].
The optimum glucose and urea concentrations were equal to the high level (+1), which raises questions about the model’s validity at higher concentrations. To validate the model and to test the influence of elevated levels of glucose and urea, triplicates of extra five runs were tested (Table 12). The results suggest that the models are valid within the experimental space. Increasing the urea concentration (0.9 %, w/v) sustains the validity of the models with relatively low standard error. Increasing the glucose concentration (5 %, w/v) reflects the validity of the growth and lipase models with relatively high standard error values. The elevated glucose level led to inaccurate predictions for the transesterification model, showing explicit limitation, which could result from the hindering effect of methanol on the cell wall integrated during the reaction [48].
4 Conclusion
A. flavus NDA04a was isolated from soil and selected because it showed a relatively high catalytic transesterification capability compared to other isolates; then, it was randomly mutated, and the mutant showed higher lipase activity was selected for this study [14]. To our knowledge, this might be the 1st or among the very early reports on the optimization of A. flavus cultivation conditions to maximize the biomass capability to catalyze the transesterification of TG to biodiesel.
The biomass lipase activity and transesterification capability were mainly the same in both culture media A and B, and medium A had 18.5 % more biomass. Hence, medium A (glucose-polypeptone) was selected for further optimization. Submerged fermentation at 200 rpm increased the growth, lipase activity, and transesterification capability by 3, 4.9, and 2.5 folds, respectively, compared to static conditions. Olive oil was the most potent lipolytic activity inducer. As a replacement for polypeptone, urea improved lipase activity by 12.8 % and transesterification capability by 4.6 %, while the growth was reduced to 13.75 g L−1.
Plackett Burman screening showed that KH2PO4 concentration, urea concentration, and glucose concentration had the highest significant main effects on biomass transesterification capability and lipase activity and significantly affected the growth; therefore, they were selected for further optimization. Response surface methodology (RSM) was applied using central composite design (CCD) for further optimization. CCD models for growth, biomass lipase activity, and transesterification capability were checked for adequacy and validated. The optimum concentrations for growth and biomass transesterification were glucose (4.5 %, w/v), urea (0.7 %, w/v), and KH2PO4 (0.9 %, w/v), and those for lipase activity of biomass were glucose (4.4 %, w/v), urea (0.7 %, w/v), and KH2PO4 (2.1 %, w/v). Overall, the optimization process increased the biomass catalytic lipid modification capability of the produced biomass, the transesterification capability of the produced biomass, in terms of FAME percentage, was improved by 2.1 folds from 40.51 to 86.71%, and the biomass lipase activity was enhanced by 2.3 folds from 9.69 to 22.14 U/g.
Data availability
The datasets generated and analyzed during the current study and not included in this published article are available from the corresponding author upon reasonable request.
References
de Carvalho CCCR (2017) Whole cell biocatalysts: essential workers from nature to the industry. Microb Biotechnol 10:250–263. https://doi.org/10.1111/1751-7915.12363
Nett JE, Sanchez H, Cain MT, Andes DR (2010) Genetic basis of Candida biofilm resistance due to drug-sequestering matrix glucan. J Infect Dis 202:171–175. https://doi.org/10.1086/651200
Beauvais A, Latgé JP (2018) Special issue: fungal cell wall. J Fungi 4. https://doi.org/10.3390/JOF4030091
Fukuda H, Kondo A, Tamalampudi S (2009) Bioenergy: sustainable fuels from biomass by yeast and fungal whole-cell biocatalysts. Biochem Eng J 44:2–12. https://doi.org/10.1016/J.BEJ.2008.11.016
Gupta VK, Kubicek CP, Berrin JG et al (2016) Fungal enzymes for bio-products from sustainable and waste biomass. Trends Biochem Sci 41:633–645
Ravindran R, Jaiswal AK (2016) Microbial enzyme production using lignocellulosic food industry wastes as feedstock: a review. Bioengineering 3:30
Park HS, Jun SC, Han KH et al (2017) Diversity, Application, and Synthetic Biology of Industrially Important Aspergillus Fungi. Adv Appl Microbiol 100:161–202. https://doi.org/10.1016/bs.aambs.2017.03.001
Cleveland TE, Yu J, Fedorova N et al (2009) Potential of Aspergillus flavus genomics for applications in biotechnology. Trends Biotechnol 27:151–157
Sana S, Anjum AA, Yaqub T et al (2019) Enzymatic and aflatoxin production potential of Aspergillus flavus. Pak J Agric Sci 56:747–752. https://doi.org/10.21162/PAKJAS/19.6121
Tan H, Yin H (2020) Optimization and characterization of oligosaccharides production from citrus peel waste resource using Aspergillus niger 1805. J Microbiol Methods 169:105809. https://doi.org/10.1016/j.mimet.2019.105809
Alabdalall AH, Alanazi NA, Aldakeel SA et al (2020) Molecular, physiological, and biochemical characterization of extracellular lipase production by Aspergillus niger using submerged fermentation. PeerJ 8:e9425. https://doi.org/10.7717/peerj.9425
Singh V, Haque S, Niwas R et al (2017) Strategies for fermentation medium optimization: an in-depth review. Front Microbiol 7:2087. https://doi.org/10.3389/FMICB.2016.02087/BIBTEX
Beg S, Swain S, Rahman M et al (2019) Application of design of experiments (DoE) in pharmaceutical product and process optimization. Pharm Qual Des: Princ Appl 43–64. https://doi.org/10.1016/B978-0-12-815799-2.00003-4
Elhussiny NI, Khattab AENA, El-Refai HA et al (2020) Assessment of waste frying oil transesterification capacities of local isolated Aspergilli species and mutants. Mycoscience 61:136–144. https://doi.org/10.1016/j.myc.2020.01.003
Jensen RG (1983) Detection and determination of lipase (acylglycerol hydrolase) activity from various sources. Lipids 18(9):650–657. https://doi.org/10.1007/BF02534677
Salvatierra HN, Regner EL, Baigorí MD, Pera LM (2021) Orchestration an extracellular lipase production from Aspergillus niger MYA 135: biomass morphology and fungal physiology. AMB Express 11. https://doi.org/10.1186/S13568-021-01202-Y
el Enshasy HA (2022) Fungal morphology: a challenge in bioprocess engineering industries for product development. Curr Opin Chem Eng 35:100729. https://doi.org/10.1016/J.COCHE.2021.100729
Gopinath SCB, Anbu P, Lakshmipriya T, Hilda A (2013) Strategies to characterize fungal lipases for applications in medicine and dairy industry. Biomed Res Int 2013. https://doi.org/10.1155/2013/154549
Shu ZY, Jiang H, Lin RF et al (2010) Technical methods to improve yield, activity and stability in the development of microbial lipases. J Mol Catal B Enzym 62:1–8. https://doi.org/10.1016/J.MOLCATB.2009.09.003
Putri DN, Khootama A, Perdani MS et al (2020) Optimization of Aspergillus niger lipase production by solid state fermentation of agro-industrial waste. Energy Rep 6:331–335. https://doi.org/10.1016/J.EGYR.2019.08.064
Prabaningtyas RK, Putri DN, Utami TS, Hermansyah H (2018) Production of immobilized extracellular lipase from Aspergillus niger by solid state fermentation method using palm kernel cake, soybean meal, and coir pith as the substrate. Energy Procedia 153:242–247. https://doi.org/10.1016/J.EGYPRO.2018.10.010
Tacin MV, Massi FP, Fungaro MHP et al (2019) Biotechnological valorization of oils from agro-industrial wastes to produce lipase using Aspergillus sp. from Amazon. Biocatal Agric Biotechnol 17:369–378. https://doi.org/10.1016/J.BCAB.2018.11.013
Scoma A, Coma M, Kerckhof FM et al (2017) Efficient molasses fermentation under high salinity by inocula of marine and terrestrial origin. Biotechnol Biofuels 10:1–17. https://doi.org/10.1186/S13068-017-0701-8/FIGURES/7
Facchini F, Vici AC, Pereira MG et al (2016) Enhanced lipase production of Fusarium verticillioides by using response surface methodology and wastewater pretreatment application. J Biochem Technol 6(3):996–1002
Lívanský K (1982) Buffering capacity of the chief components of nutritive media for algae. Folia Microbiol (Praha) 27:455–459. https://doi.org/10.1007/BF02876459
Yaping L, Bo Z, Kalamiyets E et al (2019) Research on response surface optimization of culture medium for antibacterial substances produced by bacillus Amyloliquefaciens GN59. ACM Int Conf Proc Ser 75–81. https://doi.org/10.1145/3314367.3314368
Naqvi SH, Khan MY, Rafiq M, Dahot MU (2012) Screening of lipase producing fungi and catalytic activity from molasses culture medium. Sindh Univ Res J - SURJ (Sci Ser) 44
Ribeiro Lima LG, Machado Gonçalves MM, Couri S et al (2019) Lipase production by Aspergillus niger C by submerged fermentation. Braz Arch Biol Technol 62:1–14. https://doi.org/10.1590/1678-4324-2019180113
Kittikun A, Cheirsilp B, Sohsomboon N et al (2021) Palm oil decanter cake wastes as alternative nutrient sources and biomass support particles for production of fungal whole-cell lipase and application as low-cost biocatalyst for biodiesel production. Processes 9:1365. https://doi.org/10.3390/PR9081365/S1
di Luccio M, Capra F, Ribeiro NP et al (2004) Effect of temperature, moisture, and carbon supplementation on lipase production by solid-state fermentation of soy cake by Penicillium simplicissimum. Appl Biochem Biotechnol 113
Salgado JM, Abrunhosa L, Venâncio A et al (2014) Integrated use of residues from olive mill and winery for lipase production by solid state fermentation with Aspergillus sp. Appl Biochem Biotechnol 172:1832–1845. https://doi.org/10.1007/S12010-013-0613-4/FIGURES/3
Krausfeldt LE, Farmer AT, Castro Gonzalez HF et al (2019) Urea is both a carbon and nitrogen source for Microcystis aeruginosa: Tracking 13C incorporation at bloom pH conditions. Front Microbiol 10:1064. https://doi.org/10.3389/FMICB.2019.01064/BIBTEX
Cihangir N, Sarikaya E (2004) Investigation of lipase production by a new isolate of Aspergillus sp. World J Microbiol Biotechnol 20(2):193–197. https://doi.org/10.1023/B:WIBI.0000021781.61031.3A
Dalmau E, Montesinos JL, Lotti M, Casas C (2000) Effect of different carbon sources on lipase production by Candida rugosa. Enzym Microb Technol 26:657–663. https://doi.org/10.1016/S0141-0229(00)00156-3
Xiang M, Zhang K, Yonggang W et al (2021) Optimization of low-temperature lipase production conditions and study on enzymatic properties of Aspergillus Niger. Iran J Chem Chem Eng 40:1364–1374. https://doi.org/10.30492/IJCCE.2021.529010.4694
Burkert JFM, Maugeri F, Rodrigues MI (2004) Optimization of extracellular lipase production by Geotrichum sp. using factorial design. Bioresour Technol 91:77–84. https://doi.org/10.1016/S0960-8524(03)00152-4
Khan M, Chaudhary A, Mukhtar H (2022) Production, purification and immobilization of triacylglycerol lipase from indigenous lipolytic microorganisms. Latin Am Appl Res - Int J 52:157–166. https://doi.org/10.52292/J.LAAR.2022.677
Sumarsih S, Hadi S, Andini DGT et al (2021) The effect of water content and carbon source addition on lipase production from Aspergillus aculeatus Ms.11 using spent coffee ground. IOP Conf Ser Earth Environ Sci 948:012055. https://doi.org/10.1088/1755-1315/948/1/012055
Shabbir A, Mukhtar H (2018) Optimization process for enhanced extracellular lipases production from a new isolate of Aspergillus terreusah-F2. Pak J Bot 50:1571–1578
Linder T (2018) Assimilation of alternative sulfur sources in fungi. World J Microbiol Biotechnol 34:51. https://doi.org/10.1007/S11274-018-2435-6
Gomes NB, Dias KB, Teixeira MFN et al (2018) <b>Medium composition and Amazonian oils for lipase production by <i>Candida viswanathii. Acta Sci Technol 40:e35088. https://doi.org/10.4025/actascitechnol.v40i1.35088
Carrazco-Palafox J, Rivera-Chavira BE, Ramírez-Baca N et al (2018) Improved method for qualitative screening of lipolytic bacterial strains. MethodsX 5:68–74. https://doi.org/10.1016/j.mex.2018.01.004
Maldonado R, Fernandes J, Burkert M et al (2014) Elucidation of the effects of inoculum size and age on lipase production by Geotrichum candidum. Biotecnol Apl 31:216–221. https://doi.org/10.1155/2013/154549
Mukhtar H, Hanif M, Nawaz A, Haq IU (2015) Studies on the lipase production by aspergillus Niger through solid state fermentation. Pak J Bot 47:351–354
Talukder MMR, Lee HZS, Low RF et al (2013) Potential use of whole cell lipase from a newly isolated Aspergillus nomius for methanolysis of palm oil to biodiesel. J Mol Catal B Enzym 89:108–113. https://doi.org/10.1016/J.MOLCATB.2013.01.001
VM GL, Krieger N, Inez Sarquis MM et al (2003) Effect of nitrogen and carbon sources on lipase production by Penicillium aurantiogriseum. Food Technol Biotechnol 41:105–110
Wu XY, Jääskeläinen S, Linko YY (1996) An investigation of crude lipases for hydrolysis, esterification, and transesterification. Enzym Microb Technol 19:226–231. https://doi.org/10.1016/0141-0229(95)00239-1
Lotti M, Pleiss J, Valero F, Ferrer P (2015) Effects of methanol on lipases: molecular, kinetic and process issues in the production of biodiesel. Biotechnol J 10:22–30. https://doi.org/10.1002/BIOT.201400158
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
GC, HA, and NE conceived and designed the research. NE conducted experiments, analyzed data, and wrote the manuscript. GC, HA, HE, SS, and YS are responsible for the supervision and revision.
Corresponding author
Ethics declarations
Ethics approval
This article contains no studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elhussiny, N.I., El-Refai, H.A., Mohamed, S.S. et al. Aspergillus flavus biomass catalytic lipid modification: optimization of cultivation conditions. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04396-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04396-2