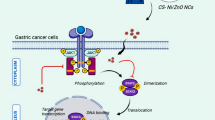
Abstract
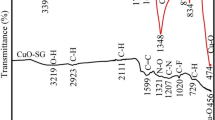
In recent years, the development of green multifunctional nanoformulations with specific anticancer activity has gained importance among scientists. In the present study, Camelia sinensis extract/CuO nanoparticles (CSE/CuO NPs) and cisplatin (Cis)-loaded CSE/CuO NPs were prepared and characterized by various TEM, XRD, FTIR, and UV-Vis techniques. The antioxidant properties of the green CSE/CuO NPs were evaluated using the CUPRIC Reducing Antioxidant Capacity (CUPRAC) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS)/HRP methods. The drug release profile of the NPs was studied at pH 7.4 and pH 5.5. Additionally, the cytotoxic effects of CSE/CuO NPs and Cis-loaded CSE/CuO NPs on MIA-PaCa-2 pancreatic cancer were evaluated using the MTT assay and compared with normal HUVEC cells. Subsequently, the apoptotic activity of CSE/CuO NPs and Cis-loaded CSE/CuO NPs on MIA-PaCa-2 cells was evaluated using Annexin-V FITC, and their effects on multi-drug resistance were determined using the ABCG2 antibody by flow cytometric analysis. Our results showed that Cis-loaded CSE/CuO NPs exhibited a pH-sensitive drug release profile. CSE/CuO NPs were highly cytotoxic to MIA-PaCa-2 pancreatic cancer cells, while exhibiting very low cytotoxicity to HUVEC cells. Moreover, CSE/CuO NPs and Cis-loaded CSE/CuO NPs were found to have higher apoptotic activity and lower drug resistance compared to free Cis. Although many studies have been conducted with CSE, this study was the first to show that CSE/CuO NPs were dominantly and selectively effective against pancreatic cancer, and resistance to Cis and its side effects could be significantly reduced by CSE-derived nanocarriers. Additionally, for the first time, the antioxidant property was investigated by applying CUPRAC and ABTS methods.
Graphical Abstract







Similar content being viewed by others
Data availability
Not applicable.
References
Moffat GT, Epstein AS, O’Reilly EM (2019) Pancreatic cancer—a disease in need: Optimizing and integrating supportive care. Cancer 125:3927–3935. https://doi.org/10.1002/CNCR.32423
Heinemann V (2002) Gemcitabine in the treatment of advanced pancreatic cancer: a comparative analysis of randomized trials. Semin Oncol 29:9–16. https://doi.org/10.1016/S0093-7754(02)70021-3
Tchounwou PB, Dasari S, Noubissi FK et al (2021) Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J Exp Pharmacol 13:303. https://doi.org/10.2147/JEP.S267383
Conde VR, Alves MG, Oliveira PF, Silva BM (2015) Tea (Camellia sinensis (L.)): a Putative Anticancer Agent in Bladder Carcinoma? Anti-Cancer Agents Med Chem 15(1):26–36
Article R, Dias RT, Tomás G, Teixeira NF et al (2013) White tea (Camellia sinensis (L.)): an-tioxidant properties and beneficial health effects. Int J Food Sci 2:19–26. https://doi.org/10.19070/2326-3350-130005
Liu J, Xing J, Fei Y (2008) Green tea (Camellia sinensis) and cancer prevention: a systematic review of randomized trials and epidemiological studies. Chin Med 3:12. https://doi.org/10.1186/1749-8546-3-12
Imran A, Butt MS, Xiao H et al (2019) Inhibitory effect of black tea (Camellia sinensis) theaflavins and thearubigins against HCT 116 colon cancer cells and HT 460 lung cancer cells. J Food Biochem 43:e12822. https://doi.org/10.1111/JFBC.12822
Keckstein S, Tilgener C, Jeschke U et al (2022) Effects of matcha tea extract on cell viability and peroxisome proliferator-activated receptor γ expression on T47D breast cancer cells. Arch Gynecol Obstet 1:1–9. https://doi.org/10.1007/S00404-021-06381-4/FIGURES/4
Xu L, Zhang L, Ren D et al (2022) Green synthesis of Cu/Fe3O4 nanoparticles using green tea extract: evaluation of its catalytic activity, antioxidant and anti-colon cancer effects. Inorg Chem Commun 144:109927. https://doi.org/10.1016/J.INOCHE.2022.109927
Bedrood Z, Rameshrad M, Hosseinzadeh H (2018) Toxicological effects of Camellia sinensis (green tea): a review. Phytother Res 32:1163–1180. https://doi.org/10.1002/PTR.6063
Haghparasti Z, Mahdavi Shahri M (2018) Green synthesis of water-soluble nontoxic inorganic polymer nanocomposites containing silver nanoparticles using white tea extract and assessment of their in vitro antioxidant and cytotoxicity activities. Mater Sci Eng C 87:139–148. https://doi.org/10.1016/J.MSEC.2018.02.026
Wang Y, Yi S, Lu R et al (2021) Preparation, characterization, and 3D printing verification of chitosan/halloysite nanotubes/tea polyphenol nanocomposite films. Int J Biol Macromol 166:32–44. https://doi.org/10.1016/J.IJBIOMAC.2020.09.253
Dutta GK, Karak N (2019) Waste brewed tea leaf derived cellulose nanofiber reinforced fully bio-based waterborne polyester nanocomposite as an environmentally benign material. RSC Adv 9:20829–20840. https://doi.org/10.1039/C9RA02973G
Karakuş S, Baytemir G, Özeroğlu C, Taşaltın N (2022) An ultra-sensitive smartphone-integrated digital colorimetric and electrochemical Camellia sinensis polyphenols encapsulated CuO nanoparticles-based ammonia biosensor. Inorg Chem Commun 143:109733. https://doi.org/10.1016/J.INOCHE.2022.109733
Shaheen M, Kalwar NH, Intisar A et al (2021) Efficient surfactant modified copper oxide nanoparticles for solar light driven water purification. Opt Mater (Amst) 122:111688. https://doi.org/10.1016/J.OPTMAT.2021.111688
Meydaner TF, Özdemir O, Afsin Kariper I (2016) The effects of ph on structural and optıcal characterızatıon of ıron oxıde thın fılms. 24(04):1750051. https://doi.org/10.1142/S0218625X17500512
Kariper IA (2016) Production and characterization of TeIx (x: 2, 4) thin films: optical, structural properties and effect of porosity. Mater Des 106:170–176. https://doi.org/10.1016/J.MATDES.2016.05.122
Apak R, Güçlü K, Özyürek M, Karademir SE (2004) Novel total antioxidant capacity ındex for dietary polyphenols and vitamins C and E, using their cupric ıon reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem 52:7970–7981. https://doi.org/10.1021/JF048741X
Arnao MB, Cano A, Acosta M (2001) The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem 73:239–244. https://doi.org/10.1016/S0308-8146(00)00324-1
Karakuş S, Baytemir G, Özeroğlu C, Taşaltın N (2022) An ultra-sensitive smartphone-integrated digital colorimetric and electrochemical Camellia sinensis polyphenols encapsulated CuO nanoparticles-based ammonia biosensor. Inorg Chem Commun 143:109733. https://doi.org/10.1016/J.INOCHE.2022.109733
Rajgovind SG, Kr DG et al (2015) Pterocarpus marsupium derived phyto-synthesis of copper oxide nanoparticles and their antimicrobial activities. J Microb Biochem Technol 7:1–5. https://doi.org/10.4172/1948-5948.1000195
Pȩkal A, Drózdz P, Biesaga M, Pyrzynska K (2012) Screening of the antioxidant properties and polyphenol composition of aromatised green tea infusions. J Sci Food Agric 92:2244–2249. https://doi.org/10.1002/JSFA.5611
Atoui AK, Mansouri A, Boskou G, Kefalas P (2005) Tea and herbal infusions: their antioxidant activity and phenolic profile. Food Chem 89:27–36. https://doi.org/10.1016/J.FOODCHEM.2004.01.075
Jiang Y, Jiang Z, Ma L, Huang Q (2021) Advances in nanodelivery of green tea catechins to enhance the anticancer activity. Molecules 26. https://doi.org/10.3390/MOLECULES26113301
Li F, Wang F, Yu F et al (2008) In vitro antioxidant and anticancer activities of ethanolic extract of selenium-enriched green tea. Food Chem 111:165–170. https://doi.org/10.1016/J.FOODCHEM.2008.03.057
Wang Y, Yi S, Lu R et al (2021) Preparation, characterization, and 3D printing verification of chitosan/halloysite nanotubes/tea polyphenol nanocomposite films. Int J Biol Macromol 166:32–44. https://doi.org/10.1016/J.IJBIOMAC.2020.09.253
Ji N, Dong C, Jiang J (2022) Evaluation of antioxidant, cytotoxicity, and anti-ovarian cancer properties of the Fe3O4@CS-Starch/Cu bio-nanocomposite. Inorg Chem Commun 140:109452. https://doi.org/10.1016/J.INOCHE.2022.109452
Hosny M, Fawzy M, Eltaweil AS (2022) Phytofabrication of bimetallic silver-copper/biochar nanocomposite for environmental and medical applications. J Environ Manage 316:115238. https://doi.org/10.1016/J.JENVMAN.2022.115238
Fujioka K, Iwamoto T, Shima H et al (2016) The powdering process with a set of ceramic mills for green tea promoted catechin extraction and the ROS ınhibition effect. Molecules 21. https://doi.org/10.3390/MOLECULES21040474
Widatalla HA, Yassin LF, Alrasheid AA et al (2022) Green synthesis of silver nanoparticles using green tea leaf extract, characterization and evaluation of antimicrobial activity. Nanoscale Adv 4:911–915. https://doi.org/10.1039/D1NA00509J
Karabatak A, Danışman-Kalındemirtaş F, Tan E et al (2022) Kappa carrageenan/PEG-CuO nanoparticles as a multifunctional nanoplatform: digital colorimetric biosensor and anticancer drug nanocarrier. Applied Physics A 128(8):661. https://doi.org/10.1007/S00339-022-05802-8
Maulana I, Fasya D, Ginting B (2022) Biosynthesis of Cu nanoparticles using Polyalthia longifolia roots extracts for antibacterial, antioxidant and cytotoxicity applications. 37(13):2517–2521
Kong F, Liu X, Zhou Y et al (2020) Downregulation of METTL14 increases apoptosis and autophagy induced by cisplatin in pancreatic cancer cells. Int J Biochem Cell Biol 122:105731. https://doi.org/10.1016/J.BIOCEL.2020.105731
Galluzzi L, Senovilla L, Vitale I et al (2011) Molecular mechanisms of cisplatin resistance. Oncogene 31(15):1869–1883. https://doi.org/10.1038/onc.2011.384
Basu A, Krishnamurthy S (2010) Cellular responses to Cisplatin-induced DNA damage. J Nucleic Acids 2010:201367. https://doi.org/10.4061/2010/201367
Nematbakhsh M, Ashrafi F, Pezeshki Z et al (2012) A histopathological study of nephrotoxicity, hepatoxicity or testicular toxicity: which one is the first observation as side effect of Cisplatin-induced toxicity in animal model? J Nephropathol 1:190. https://doi.org/10.5812/NEPHROPATHOL.8122
Wu J, Ye J, Kong W et al (2020) Programmed cell death pathways in hearing loss: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 53:e12915. https://doi.org/10.1111/CPR.12915
Bhardwaj JK, Paliwal A, Saraf P, Sachdeva SN (2022) Role of autophagy in follicular development and maintenance of primordial follicular pool in the ovary. J Cell Physiol 237:1157–1170. https://doi.org/10.1002/JCP.30613
Bansal KK, Bhardwaj JK, Saraf P et al (2020) Synthesis of thiazole clubbed pyrazole derivatives as apoptosis inducers and anti-infective agents. Mater Today Chem 17:100335. https://doi.org/10.1016/J.MTCHEM.2020.100335
Jan R, Chaudhry G (2019) Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv Pharm Bull 9:205. https://doi.org/10.15171/APB.2019.024
Kamal R, Kumar R, Kumar V et al (2020) Diacetoxy iodobenzene mediated regioselective synthesis and characterization of novel [1,2,4]triazolo[4,3-a]pyrimidines: apoptosis inducer, antiproliferative activities and molecular docking studies. J Biomol Struct 39:4398–4414. https://doi.org/10.1080/07391102.2020.1777900
Bansal KK, Bhardwaj JK, Saraf P et al (2020) Synthesis of thiazole clubbed pyrazole derivatives as apoptosis inducers and anti-infective agents. Mater Today Chem 17:100335. https://doi.org/10.1016/J.MTCHEM.2020.100335
Gimenez-Bonafe P, Tortosa A, Perez-Tomas R (2009) Overcoming drug resistance by enhancing apoptosis of tumor cells. Curr Cancer Drug Targets 9:320–340. https://doi.org/10.2174/156800909788166600
Khunweeraphong N, Stockner T, Kuchler K (2017) The structure of the human ABC transporter ABCG2 reveals a novel mechanism for drug extrusion. Sci Rep 7(1):13767. https://doi.org/10.1038/s41598-017-11794-w
Wong RSY (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin. Cancer Res 30:1–14. https://doi.org/10.1186/1756-9966-30-87/TABLES/3
Alhakamy NA, Ahmed OAA, Aldawsari HM et al (2019) Encapsulation of lovastatin in zein nanoparticles exhibits enhanced apoptotic activity in HepG2 cells. Int J Mol Sci 20:5788. https://doi.org/10.3390/IJMS20225788
Danışman-Kalındemirtaş F, Kariper İA, Erdemir G et al (2022) Evaluation of anticancer effects of carboplatin–gelatin nanoparticles in different sizes synthesized with newly self-assembly method by exposure to IR light. Sci Rep 12(1):10686. https://doi.org/10.1038/s41598-022-15051-7
Kucuksayan E, Bozkurt F, Yilmaz MT et al (2021) A new combination strategy to enhance apoptosis in cancer cells by using nanoparticles as biocompatible drug delivery carriers. Sci Rep 11:1–19. https://doi.org/10.1038/s41598-021-92447-x
Shinde VR, Revi N, Murugappan S et al (2022) Enhanced permeability and retention effect: a key facilitator for solid tumor targeting by nanoparticles. Photodiagnosis Photodyn Ther 39:102915. https://doi.org/10.1016/J.PDPDT.2022.102915
Nakamura Y, Mochida A, Choyke PL, Kobayashi H (2016) Nanodrug delivery: ıs the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug Chem 27:2225–2238. https://doi.org/10.1021/ACS.BIOCONJCHEM.6B00437/ASSET/IMAGES/MEDIUM/BC-2016-00437T_0005.GIF
Author information
Authors and Affiliations
Contributions
Ferdane Danışman Kalındemirtaş: conceptualization, methodology, validation, formal analysis, and investigation. Esra Sert: methodology, formal analysis, and investigation. Ezgi Tan: methodology, formal analysis, and investigation. Esin Akyüz: methodology, analysis, and/or interpretation of data. Selcan Karakuş: conceptualization, methodology, validation, formal analysis, and investigation.
Corresponding authors
Ethics declarations
Ethical approval
Any of the writers conducted no human or animal experiments in this publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Danışman-Kalındemirtaş, F., Sert, E., Tan, E. et al. Potential multifaceted applications of cisplatin-loaded Camellia sinensis extract/CuO nanoparticles in cytotoxic and apoptotic effects. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04222-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04222-9