Abstract
This study aimed to investigate waste-tea’s pyrolysis kinetics and thermodynamics to assess its potential for thermochemical processes. In this study, three primary samples of tea-waste are prepared for investigation: raw, torrefied at 200 °C, and torrefied at 600 °C. Under a nitrogen environment, thermogravimetric analyses (TGA) were conducted at seven heating rates (10, 15, 20, 25, 30, 35, and 40 °C/min) to investigate the effect of heating rates on the kinetic parameters at temperatures ranging from 27 to 1000 °C. Using seven heating rates was beneficial to take advantage of multiple heating rates techniques alongside single heating rate techniques. These heating rates were combined, forming four heating rate groups (HRG). The pyrolysis kinetic parameters are determined using two model-fit-methods, direct Arrhenius and Coats-Redfern methods, and two model-free methods, Flynn-Wall-Ozawa (FWO) and Kissinger-Akahira-Sunose (KAS) methods. Thermodynamic data comprising ΔH, ΔG, and ΔS are addressed. The X-ray fluorescence (XRF) and Fourier transform infrared (FTIR) spectrum are used to assess the presence of natural minerals in tea-waste. The results indicated that the tea-waste material has the potential to produce syngas. The torrefaction process at 600 °C shows a 53% increase in the energy content compared to the raw biomass. The Coats-Redfern is shown to be more reliable than the direct Arrhenius method. The activation energy (Ea) witnesses rising with the heating rate (β) from Ea = 55.27 kJ mol−1 at β = 10 °C min−1 to 60.04 kJ mol−1 at β = 40 °C min−1 for raw tea-waste using Coats-Redfern method. For model-free approaches, the minimum activation energy values of the raw tea-waste samples are 82 kJ/mol for FWO and 78 kJ/mol for KAS, whereas the peak values are 420 kJ/mol for KAS and 411 kJ/mol for FWO. A comparison of the effect of heating rate groups for FWO method in raw material case indicated that HRG1 has the maximum activation energy average value. The resulting values of HRG1, HRG2, HRG3, and HRG4 were 269 kJ/mol, 145 kJ/mol, 174 kJ/mol, and 202 kJ/mol, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Considering the growing concerns about the predicted depletion of the present fossil fuels’ reserves within the next several decades and the harmful emissions, it has become essential to ensure the availability of more reliable and environmentally acceptable energy resources [1, 2]. Because of environmental and economic considerations, biomass is one of the best efficient renewable energy resources [3,4,5]. Moreover, biomass has a high potential energy density and is portable and storable material [6]. Biomass includes raw agricultural products, food industry waste, and wood waste. Additionally, biomass may be transformed into high potential energy types alongside electricity generation [7].
Although biomass is available from many sources, its energy content is considered medium compared with fossil fuels. Hence, there are several ways to elevate the energy density of biomass. Various thermochemical procedures such as combustion, pyrolysis, gasification, torrefaction, and hydrothermal carbonization are vital approaches for biomass treatment, yielding a variety of desired components. Torrefaction and carbonization procedures are utilized to develop a high-carbon content fuel that upgrades the energy level [8]. Torrefaction, heating material to high temperature (200–300 °C) for a specific time, is one of the most effective methods for this purpose. Therefore, there are methods to evaluate raw and thermally treated biomass characteristics. Thermogravimetric analysis (TGA) is an effective approach to studying biomass chemical characteristics [9, 10].
The agro-food industry can potentially be one of the most critical biomass resources. Moreover, millions of tons of agro-waste are left annually, which causes environmental problems. Thus, utilizing these wastes as biomass resources has a double-beneficial step towards energy and environmental problems. Focusing on Egypt as a model, Egypt is a nation with considerable agriculture, and crop residual waste abundant in Northern Egypt comprises a substantial quantity of dense and moist resources. It is projected that more than 20 million tons of agricultural waste are produced annually, posing significant disposal issues. The most recent legislation enacted by the Egyptian Environmental Authority governing particulate matter emissions from burning would require waste to be disposed of by recycling or landfilling. Therefore, it is essential to discover and utilize for such waste products [11].
All thermochemical plant design (carbonization, gasification, or combustion) includes the design for each piece of equipment and auxiliary sizing, which requires an understanding of the reaction kinetics or the number and kind of reactions that occur during the process. In addition to the functionality of biomass conversion with the reaction rate, it is vital to analyze the reactions that occur and their kinetic parameters, such as activation energy and the pre-exponential factor. These parameters can be obtained either using model-free or model-fitting techniques.
The kinetic study is to provide information about kinetic mechanisms for chemical reactions and to determine kinetic parameters, pre-exponential Arrhenius factor, activation energy, and reaction order(s). The kinetic parameters are considered the director criteria used as inputs for a complete gasifier/carbonization simulation [12]. Two main approaches of kinetic analysis are commonly used in the thermal analysis [13]. This classification resulted in model-fitting techniques in which a single heating rate is required to proceed, while model-free techniques require multiple heating rates. The latter techniques are time- and cost-consuming; however, they produce higher accuracy results. On the other hand, model-fitting methods are easy to use. Regarding model-fitting methods, the most popular approaches are direct Arrhenius (DA) and Coats and Redfern (CR) models, whereas Kissinger-Akahira-Sunose (KAS) and Flynn-Wall-Ozawa (FWO) are the most effective models in model-free methods.
Research needs to investigate the most suitable biomass thermal characterizations using sort of these methods with different operating parameters. Table 1 summarizes research that studied the effect of the type of biomass, temperature range, heating rate values, and kinetic models used. The reviewed studies covered neither the thermokinetic investigation of tea-waste nor the comparative kinetic investigation by all methods. Moreover, the empirical modeling for precise determination of kinetic analysis for this material is lacking from the relevant research [14,15,16,17,18,19,20,21,22,23,24,25].
The current work provides, to the best of the authors’ knowledge, the first study that investigates the effect of different multiple heating rates on the kinetic parameters of torrefied tea-waste. In this regard, a comprehensive kinetic study with single and multiple heating rates on torrefaction treatments is carried out to find the best method for producing biochar from tea-waste. Studying the temperature effect on TGA curves and kinetic parameters is one of the primary objectives of this study. A thorough examination of raw and torrefied tea-waste at 200 °C (T200) and 600 °C (T600) critical physical and chemical characteristics is presented, including ultimate, proximate analysis, and heating values. Moreover, a full comparison between kinetic models was carried out. The DA and CR model-fitting approaches and FWO and KAS model-free approaches are considered.
2 Materials and methodology
2.1 Materials
The considered quantity of tea-waste is gathered from a local processing factory. A 7.5 kg of biomass is used in the sieving process to ensure sufficient raw material for the targeted tests and experimental work. The particle size distribution was determined using Sieve Shaker (model: analysette 3" SPARTAN pro. Fritsch GmbH, Germany) with particle size detection range of 250 to 840 μm. The small particle size minimizes the experimental operational time and cost since there is no need for biomass crushing. Moreover, because of the small pyrolysis particles, internal conduction heat transfer resistance can be neglected [26].
2.2 Experimental methodology
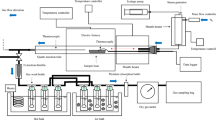
Thermochemical treatment processes for the samples are performed using AMF-25 N 3kW muffle furnace. Firstly, all cases were thermally heated at 105 °C for a whole day to ensure that all water was vaporized from the sample. The heating process was carried out utilizing heater (model ED 23 Model, BINDER GmbH, Tuttlingen, Germany). The temperature values of the torrefaction process are 200 °C and 600 °C for 1 h of residence time. Hence, there are mainly three samples: raw, T200, and T600. This covers torrefaction and carbonization temperatures of 200 and 600 °C, respectively.
The dried tea-waste was first put in a ceramic crucible. The crucible was then inserted in a 0.35-l stainless-steel reactor manufactured to resist high-temperature range and tight enough to maintain an inert environment in the experiments. Initially, the reactor was purged with pure nitrogen to avoid the existence of air or oxygen. Then, the container was blasted with a high-velocity stream of nitrogen before being placed in a muffle furnace. The temperature controller of the furnace was adjusted to the desired temperature before heating was initiated. The reactor has a K-type thermocouple, enabling accurate temperature measurement. The stainless-steel reactor has two ports for purging purposes and maintaining it at constant pressure. The residence time was calculated after reaching the desired temperature. After 1 h passed, the system was shut down to drop the temperature, preventing oxidation until the temperature reached room temperature [27]. The sample was finally placed in a sealed zip-lock bag for upcoming examinations.
2.3 Characterization
Biomass characteristics, including ultimate analysis (elemental analysis), proximate analysis, and heating values, were summarized in this section. The ultimate analysis is carried out using Vario Micro Elementar based on ASTM D5291 approach. The experiment’s outcomes reveal the biomass sample elemental analysis percentages of four main elements on a dry basis. The H, N, C, and S elements are considered the primary elements. Oxygen was calculated by subtraction based on the dry-ash-free method.
The higher heating values (HHV) for raw tea-waste, T200, and T600 were measured using oxygen bomb calorimeter (model: LBC-C22, Labtron Equipment Ltd., Camberley, UK). The energy yield (EY), enhancement factor (EF), and mass yield (MY) are estimated using the following equations:
where mraw and mf are the mass of raw and torrefied waste, respectively.
FTIR instrument (model: Alpha II, Bruker, Germany) is used to effectively carry out a Fourier transform infrared (FTIR) analysis.
2.4 TGA and DTG
The TGA and the differentiation of the TGA curve, DTG, were conducted using simultaneous thermal analyzer equipment (model: SDT650, New Castle, USA). The sample temperature was increased gradually, starting initially from the ambient conditions until peaking and ending at 1000 °C. Aiming to conduct slow pyrolysis, lower heating rates with longer residence time, 60 min, were taken into consideration. The experiments are conducted at seven heating rates (β = 10–40 °C min−1, step of 5 °C min−1). The TG, DTG, and the second derivative of the TG (DDTG) for every single case of raw and torrefied materials at different heating rates are graphed by different combinations in the “Results” section.
2.5 Kinetics analysis
Determining the reaction kinetics yields critical data on biomass degradation and is highly beneficial for the construction and sizing of commercial reactors [28]. Furthermore, it generates a simulation analysis that can be utilized for demonstrating the procedure and helps illuminate the chemical reactivity. Determining reaction kinetics facilitates calculating thermal process expenditures [29]. To utilize kinetic approaches, it is necessary to identify the onset and offset points, which are determined in accordance with ISO 11357–1:2010–03 [26]. The thermochemical process of biomass is relatively complicated.
Although multi-step kinetics provides more accurate results in many cases, none of the multi-step methods is as computationally simple as those used to analyze single-step kinetics. Often, single-step kinetics may be simplified to linear regression, which is typically uncomplicated computationally [30]. In addition, biomass thermal decomposition involves several processes that can occur concurrently or independently; thus, it is expressed by several complicated reactions. In certain applications, such as reactor analysis and simulation, a single-step reaction is used to simplify the process [31, 32]. During torrefaction, the specific reaction mechanism that is widely used is as follows [33]:
Biomass degradation, dα/dt, is calculated as [34]:
where α is defined as the mass conversion which represents the ratio between the mass reduction at time t and the maximum mass reduction for this sample.
The differential mechanism function f(α) is expressed by Eq. (6)
where the power n is the order of the reaction, and this represents the most typical nth-order reactions throughout biomass, indicating that the quantity of the left chemical constituents affects the reaction rate.
The rate constant, K, can be calculated by Arrhenius equation as:
where A, E, R, and T are pre-exponential factor (min−1), the activation energy (kJ/mol), the ideal gas constant (kJ/mol), and the temperature (K), respectively.
By combining Eqs. (5) and (7):
where (β = dT/dt) is the non-isothermal heating rate.
By integrating the previous equation for α = 0, T from 0 to T [35],
The component Q(x) in Eq. (11) has no analytical solution [35]. Thus, it can be approximated numerically using the methods described in the following sections.
As a kinetic parameter, the activation energy was calculated from a non-isothermal TGA. Several models were used to solve Eq. (11), as there are model-based and model-free methods. In the upcoming sections, all governing equations of these techniques will be discussed.
2.5.1 Model-fitting approaches
Direct Arrhenius and integral Coats-Redfern methods are two approaches for calculating the kinetic parameters: activation energy and frequency factor [36, 37]. Both models are generally used the curve fitting technique to produce a straight-line equation [36] :
where a is the slope and b is the y-axis interception.
The TGA curve results are used for determining the location of the DTG peaks. Then, the subsequent calculations are made mainly inside the peak range [27, 38, 39].
To identify the optimal model, the greatest R2 (Eq. (13)) or minimal mean square error (MSE) (Eq. (14)) and average absolute deviation (AAD) (Eq. (15)) were utilized as selection criteria.
where \({\hat{rv}}_i\) is the ith value estimated by the model, \(\overline{rv}\) is the mean actual value, rvi is the ith actual value, and ϑ is the number of parameters calculated through the regression analysis.
Arrhenius model
For the direct Arrhenius method, the logarithmic is determined for both halves of the formula in Eq. (10), leading to the following equation:
To determine the appropriate order of the reaction (n), Y vs. X graphs are created where n = (0.1–1.5 with 0.1 step). All plots are fitted with straight lines, and the R2 (coefficient of determination) with the highest value is selected. The slope = − E/R, where E can be obtained. In addition, the y-axis intercept equals b = ln A/β is used to calculate the pre-exponential factor (A).
Coats and Redfern model
Coats-Redfern model is applied using the produced data from TGA. The following governing equations are employed to conduct this research [40, 41].
The term \(\frac{2\textrm{RT}}{\textrm{E}}\) in the previous equations can be neglected compared with 1; hence, \(\frac{\textrm{AR}}{\beta \textrm{E}}\left(1-\frac{2\textrm{RT}}{\textrm{E}}\right)\) can be simplified to be \(\frac{\textrm{AR}.}{\beta \textrm{E}}\). Then, Eqs. (14) and (15) would be expressed in the following forms:
The previous equations were plotted for n = (0.1 to 1.5 with 0.1 as a step). In addition, the slope and the intercept values were at the maximum value of R2.
2.5.2 Model-free approaches
Iso-conversational methods that suppose the torrefaction process involve an unlimited number of separate and concurrent reactions are part of the model-free techniques [42]. The (FWO) model, which is an integrated framework developed by Ozawa, Flynn, and Wall [43, 44], and the KAS model are recognized as two of the most significant models utilized in this study.
Flynn-Wall-Ozawa (FWO)
The FWO method is considered one of the most validated kinetic parameter calculation approaches [45]. It relies on a linear relation, as discussed in the model-fitting methods, for a given range of conversion fraction α = 0.05: 0.05: 0.95 at multiple heating rates. This study uses seven heating rates (β = 10, 15, 20, 25, 30, 35, and 40 °C/min) in four combinations to determine the optimum case. The combination notations used for heating rates are as shown in Table 2.
The FWO method is based on the following equations [19]:
where g(α) is calculated from the following equation:
Estimating the value of E is attainable from Eq. (18). One of the FWO model’s benefits is that the activation energy (E) can be accurately approximated based on (α) regardless existing previous reaction process experience [46, 47].
Kissinger-Akahira-Sunose (KAS)
The KAS method is the most widely used technique for calculating the activation energy of thermally activated processes with multiple heating rates evaluated by the DTG [48]. It is a derivative approach that relies on the Coats-Redfern procedure [49,50,51]:
2.6 Analysis of thermodynamics
Thermodynamic detailed parameters, including A (pre-exponential factor), ΔH (total enthalpy change), ΔG (free Gibbs energy), and ΔS (total entropy change), are determined utilizing the following equations [28, 52, 53]:
where Tm, KB, and h are TG temperature at maximum DTG, Boltzmann constant, and Plank constant, respectively.
3 Results and discussion
3.1 Ultimate analysis
Table 3 illustrates the ultimate analysis that was carried out for tea-waste samples. The data reveals that carbon element is prominent, particularly with high-temperature samples. In general, this study’s carbon percentage was the highest compared to quince waste, pectin-free quince waste [23], and camel manure [25]. The carbon percentage was 45.49 ± 0.07%, 45.52 ± 1.1%, and 28% for quince waste, pectin-free quince waste, and camel manure, respectively, whereas the value was 51.89% for tea-waste. The mass ratio has ranged from 51.89 to 74.69% of the total mass. Then, oxygen in the second level ranges from 37.89 to 18.98%, followed by hydrogen and nitrogen coming in equal proportions (2.5–6%). The sulfur portion has negligible quantities (less than 0.3). This could be attributed to the decarboxylation and dehydroxylation processes that occur during the decomposition of hemicellulose, which yields a significant quantity of water, carbon dioxide, carbon monoxide, and methane [29]. The results match with other studies which were carried out on thermally treated rice husks [54] and cotton stalks [55]. Chen et al. [54] found that carbon percentage of rice husks increased constantly from 40.14% at raw case to 49.81% with increasing the torrefaction temperature to 300 °C. Moreover, oxygen percentage decreased clearly from 39.73% at raw rice husk to 21.81% with increasing the torrefaction temperature to 300 °C. The findings also show that biomass’s nitrogen percentage increases and declines marginally as the heating temperature rises. This is aligned with the conclusions of Cantrell et al. [56], who found that the nitrogen concentration increased when the torrefaction temperature reached 350 °C, and then the percentage reduced at higher temperature levels. In the current study, nitrogen concentration rose from 4.41 in the raw material sample to 4.72% at 200 °C, and then, it sharply reduced to 3.62% at 600 °C. The maintained nitrogen in the material is referred to as “Fuel N” and appears as N–H and N-C bonding. Thus, less energy is required to activate these bonds [57].
The EY, EF, MY, and heating values are obtained with temperature-changing parameters. As illustrated in Table 4, the temperature is shown to have a negative impact on both MY and EY, while it shows a positive effect on the EF. The reduction in MY is very sharp from the raw material case to the lowest value among all examined samples at 600 °C, which recorded 39.25%, passing by 200 °C torrefaction, which witnessed 89.72%. It is easily recognized that the EF is increased by elevating the temperature by more than 50% of its initial value. This direct relation between temperature and heating value is primarily due to boosting the carbon percentage in biomass by elevating the temperature vaporizing most elements that poorly contribute to energy content.
3.2 XRF analysis
Table 5 presents the metals that existed in the considered samples using Rigaku (model: NEX CG EDXRF, Japan). Rising the torrefaction temperature to 600 °C enhances the potassium portion by around 40% compared with the raw state. Furthermore, deposits on the combustor’s walls can be produced when alkalis (as K) are mixed with other metals, reducing the efficiency of the combustion process [58]. Burning waste raw tea sources in the combustor would emit heavy metals into the environment, some of which include Fe concentration. Thus, eliminating the metal composition decreases the emission levels by around 40%. For instance, Fe level was eliminated from 7.73% at raw to 4.37% at T600. It could be noticed that torrefaction limits the synthesis of sulfates during burning because of partial sulfur elimination, inhibiting the internal deposition [59].
3.3 Proximate analysis
Figure 1 presents the results of the proximate analyses, including volatile matters (VM), fixed carbon (FC), ash, and fuel ratio (FC/VM). The fixed carbon ratio was increased from 26.6% at raw sample until it reached to 79% at the highest temperature case, T600. In contrast, raising the torrefaction temperature negatively impacts volatile matters, slumping to the lowest value of 6.32% at 600 °C compared with 66.2% in the raw material sample. This could be attributed to the thermal degradation of complex polymers at higher torrefaction temperatures. The fuel ratio climbs from almost 0.45 at raw material, reaching the maximum value of nearly 12.5 at 600 °C torrefaction temperature.
3.4 Thermogravimetric analysis (TGA-DTG-DDTG)
The TG, DTG, and DDTG curves are plotted for each case at the considered heating rates to examine the pyrolysis kinetics.
Figure 2 illustrates the procedure of estimating the pyrolysis parameters, including Tonset, Toffset, and Tpeak. ISO 11357-1:2010-03 states the following justifications for the temperature of onset and offset points [60]. The crystallization onset temperature, Tonset, is the crossing point of the extrapolated line with the derivational slope. The peak slope, mainly on the leading edge of the peak, as measured by DDTG, coincides with the inflectional point. The Toffset can be defined as the crossing point between the extended baseline at DTG = 0 and the curve that has a tangential to the end of the highest crystallization. The TPeak can be identified as the temperature obtained when the degradation rate DTG gets away most far from the mean and reach the lowest recorded value. Locating these kinetic parameters depending on the stated criteria for all samples is presented in Table 6. The importance of these parameters comes from their utilization in all kinetic study models to identify kinetic parameters. Tonset and Toffset are utilized particularly in kinetic calculations where TPeak is critical for thermodynamics study.
It is shown that by increasing the torrefaction temperature, a direct rising in Tonset is obtained with a fluctuating increase between T200 and raw case, whereas Tpeak does not record a considerable change. Moreover, increasing the heating rate directly affects all three temperature values, Tonset, Toffset, and TPeak. Regarding raw material, increasing heating rate (β) rises the Tonset, Toffset, and Tpeak from 191 to 212.9, from 552.6 to 576.69, and from 330 to 353.46, respectively. Similarly, with torrefied samples at 200 °C, elevating the heating rate (β) increased the Tonset, Toffset, and Tpeak from 202.29 to 216.69, from 544.53 to 578.86, and from 330 to 353.86, respectively.
Figure 3 shows the thermogravimetric (TG) curves for raw, torrefied at 200 °C (T200), and torrefied at 600 °C (T600) tea-waste with seven different heating rates for each of them. Generally, it could be noticed that, however, there is no significant effect of the heating rates in raw and T200 TG curves; a positive correlation is found between the heating rate and TG percentage in the T600 case. Moreover, for raw and 200, a shoulder is shown at 200–250 °C; however, there is no shoulder for 600 °C because of the removal of hemicellulose at 200–250 °C [4, 53]. The thermal degradation curve of raw tea-waste exhibits a higher percentage of mass losses than those for the thermally treated samples. The average TG values of the samples at 900 °C are 34%, 36%, and 86% for raw, T200, and T600, respectively. During the first half of the TG curve to 450 °C, both cases show a direct relation between heating rates and TG percentage where β = 40 °C/min recorded the highest TG data during this period compared to the lowest heating rate. This contrast takes place with the second half period (450–900 °C), where the maximum TG percent is taken place with the lowest heating rate, whereas the minimum TG percent is taken place with the highest heating rate. Regarding T600, the difference between β = 10 °C/min and β=40 °C/min is considered constant at 2.5% along the temperature range from ambient conditions until 900 °C.
The DTG thermograms of β = 10, 15, 20, 25, 30, 35, and 40 °C/min for the tea-waste case: raw, T200, and T600, are depicted in Fig. 3d, e, and f, respectively. All graphs follow the same pattern of the effect of the heating rate value on the DTG curve. The maximum DTG change is accompanied by the fastest heating rate (β = 40 °C/min), while the slowest heating rate (β = 10 °C/min) records the minimum DTG changes along all graphs. This directly results from efficient heat transfer at a rapid temperature change rate.
Concerning raw and T200, which are illustrated in Fig. 3d and e, respectively, the increased heating rate causes a shift towards higher temperature in TGA curves and increases the DTG difference from the datum line (DTG = 0%/min). Moreover, all curves experienced low changes at temperatures over approximately 550 °C, reflecting the biomass majority’s release at these temperatures. Raw and low-temperature thermally treated tea-waste witnesses high DTG difference from approximately − 3.7%/min at β = 10 °C/min to − 15.1%/min at β = 40 °C/min. This is because of a high potential material to release vapors in low-temperature treatment cases, which reflects the effect of hemicellulose and cellulose. Regarding T600, DTG curves have a double-peak theme differently from other cases with one concentrated peak, raw, and T200. Harsh operating conditions, such as a temperature of 600 °C, reduce the possibility of releasing vapors during thermogravimetric analysis because of releasing most of the vapor is during thermal treatment [8]. Thus, the DTG maximum range does not exceed −1.2%/min—almost 8% compared with raw biomass.
3.5 Kinetic study
Kinetic analyses are carried out using two model-fitting methods, direct Arrhenius and Coats-Redfern models, and two model-free methods, FWO and KAS models. Generally, the most vital parameters in the kinetic study are (Ea) and (A).
Focusing on model-fitting methods, the R2 values produced for calculating pre-exponential factor and activation energy. The R2 values using the Coats-Redfern approach are greater than those reported through utilizing the direct Arrhenius approach, as shown in Tables 7 and 8. These outcomes are consistent with the findings of the literature [8, 36, 61, 62], which gives much more reliability to using Coats-Redfern method.
Under the direct Arrhenius approach findings, the activation energy showed a slight decrease with increasing heating rates to approach the lowest value of 37.57 kJ.mol−1 and 47.84 kJ.mol−1 for raw and T200, respectively. The same data profiles are recorded for the A values. The raw material sample is a prime example where figures indicate that the A values decrease at first from approximately 1697 min−1 at β = 10 °C/min until they reach around 1225 min−1 at β = 15 °C/min before sharply increasing to 33491 min−1 at β = 40 °C/min. All these statistics are recorded with a random reaction order range between 1.2 and 1.5, with the highest R2 values of approximately 0.8.
Regarding Coats-Redfern method findings, the activation energy values indicated for the degradation of untreated and thermally treated tea-waste increased gradually with increasing heating rates, from Ea = 55 kJ/mol at β = 10 °C/min to 60 kJ/mol at β = 40 °C/min for a sample of raw tea-waste. The thermally treated sample at 200 °C for 1 h exhibits higher activation energy than the untreated sample. For instance, Ea value grew steadily from approximately 65 kJ/mol at β = 10 °C/min to nearly 69 kJ/mol at β = 40 °C/min. The same values trend is recorded for the pre-exponential factor (A). The R2 coefficients for both models (direct Arrhenius and Coats-Redfern) are compared to identify which model better matches the data with the highest R2 values around 0.934 for the Coats-Redfern model. Moreover, the values for MSE and AAD have the lowest values at maximum R2 values which indicate the high accuracy approach was utilized by Coats-Redfern approach. This agrees with Parthasarathy et al. [19] for camel manure biomass.
Figures 4 and 5 illustrate the line graphs between (1/T) on an x-axis and (ln(b)) on a y-axis at various degrees of conversion (α = 0.05: 0.95, a step of 0.05). These graphs are the results of utilizing FWO method for raw and T200 cases, respectively. The slope value equals the term (− 1.052E/R) utilized in the activation energy estimation.
Figures 6 and 7 illustrate the line graphs for ln(b/T2) and (1/T), with slopes resulting in (− E/R) values at various degrees of conversion (α = 0.05:0.05:0.95) to calculate the Ea.
Figure 8 illustrates the activation energy determined by FWO and KAS models with various heating rate groups. The variations in activation energy values are due to the different assumptions in FWO and KAS models. Regarding the raw case, the minimum activation energy values are 82 kJ/mol and 78 kJ/mol for FWO and KAS, respectively, whereas the maximum values are 411 kJ/mol and 420 kJ/mol for FWO and KAS, respectively. Similarly, for the thermally treated sample at 200 °C, the lowest activation energy values are 101 kJ/mol and 98 kJ/mol, while the maximum values are 296 kJ mol−1 and 298 kJ mol−1 for FWO and KAS, respectively. The results indicate that KAS model has a broader margin than FWO because KAS model has the minimum and the maximum activation energy values. The data illustrate that the thermally treated sample has lower average activation energy values in HRG1, HRG 3, and HRG 4, while HRG2 has the lowest minimum and average values for all cases and models.
Moreover, the effect of α on Ea values reveals that tea-waste mainly consists of lignin, cellulose, and hemicellulose. Regarding the effect of heating rate groups on the average activation energy for the raw case using FWO method, the activation energy was reduced from 269 kJ/mol at HRG1 to 144.5 kJ/mol at HRG2. Additionally, the average activation energy values for HRG3 and HRG4 are 173.9 kJ/mol and 201.8 kJ/mol, respectively.
3.6 Thermodynamic results
The output parameters from FWO and KAS models are used in the thermodynamic study of tea-waste in the raw state utilizing HRG1 (where β = 10, 15, and 20 °C/min). However, the KAS model yielded much more accuracy than the FWO model [63, 64], and both models are included for determining thermodynamic parameters. Table 9 reveals that the pre-exponential factor for the FWO approach ranged from 2.53215E+14 s−1 at α = 0.05 to 4.32659E+33 s−1 at α = 0.95, whereas the pre-exponential factor for the KAS method ranged from 3.49055E+14 s−1 at α = 0.05 to 2.39549E+34 s−1 at α = 0.95. This variation in the pre-exponential factor indicated that waste-tea had a complicated chemical nature and interaction mechanisms throughout heat degradation [65].
Change in enthalpy (ΔH) is regarded as one of the most significant thermodynamic parameters that validate the types of reactions (whether endothermic or exothermic reaction). Enthalpy for the FWO method varies from 184.02 kJ/mol at the lowest conversion value (α = 0.05) to 405.98 kJ/mol at the highest conversion value (α = 0.95), whereas the values for the KAS method vary from 185.61 kJ/mol at the lowest conversion value (α = 0.05) to 414.61 kJ/mol at the highest conversion value (α = 0.95), as indicated in Table 9. The mean values of enthalpy were determined to be 264.1 kJ/mol and 269.5 kJ/mol for FWO and KAS models, respectively. This signifies that energy is required to break down tea-waste (endothermic nature). In addition, the degradation process can be divided into three stages with the increase of conversion degrees (α): stage I, α = 0.05–0.35; stage II, α = 0.35–0.55; and stage III, α = 0.55–0.85 [53]. Thus, ΔH is increased gradually with conversion degree where the average change in enthalpy of FWO method is 205.01 kJ/mol for hemicellulose, 224.18 kJ/mol for cellulose, and 306.65 kJ/mol for lignin. This concludes that with conformity with activation energies, lignin requires the most significant heat energy to dissolve chemical bonds, followed by cellulose and hemicellulose.
The Gibbs free energy (ΔG) refers to the intensity and spontaneous chemical processes. The analysis reveals that ΔG varies slightly between 173.89 kJ mol−1 at α = 0.05 and 169.93 kJ mol−1 at α = 0.95 for the FWO approach and between 173.85 kJ mol−1 at α = 0.05 and 169.82 kJ mol−1 at α = 0.95 for the KAS method. The decrease in Gibbs free energy signifies that the energy of the reaction mechanism rises during the activated complex development. These outcomes are in great alignment with those of other biomass sources, including rubber wood [65], cattle manure [53], and camel grass [66]. For camel grass [66], ΔG value decreased from 177.91 kJ mol−1 at α = 0.1 to 173.70 kJ mol−1 at α = 0.9 using FWO method and decreased from 177.21 kJ mol−1 at α = 0.1 to 173.78 kJ mol−1 at α = 0.9 using KAS method.
The change in entropy (ΔS) reflected the material disorder [65]. Table 9 Illustrates that the entropy change values (ΔS) for raw waste-tea using FWO method increase from 16.51 J mol−1 K−1 at α = 0.05 to 384.69 J mol−1 K−1 at α = 0.95. Increasing change in entropy with a higher conversion degree shows that the biomass is more reactive as the temperature increases. The reactivity is significant, and the reaction mechanism will generate the activated complex more rapidly. Consequently, the greater the change entropy (ΔS), the greater the material’s reactivity.
3.7 FTIR analysis
The surface FTIR fluctuation in raw and torrefied tea-waste at 200 °C and 600 °C is analyzed using FTIR data, shown in Fig. 9. The FTIR spectra, however, have undergone observable changes because of the thermal treatment at different temperatures. As the thermally treated temperature goes up, the FTIR spectra of polymers, particularly celluloses and hemicellulose, undergo considerable changes, with the most notable changes happening between 1650 and 800 cm−1. Table 10 explains the FTIR peaks for torrefied samples based on the main biomass components (celluloses, hemicelluloses, and lignin). At 3292 cm−1 for raw and 3296 cm−1 for T200, the first peak demonstrates the dissolution of chemical bonds between the O–H bonds and cellulose. Dehydroxylation occurs during the devolatilization of hemicellulose and cellulose, as evidenced by the initial increase in transmittance followed by a sharp decrease by elevating torrefaction temperatures. Shifting peaks from raw to T200 is noticed, forming much more peaks. Moreover, torrefaction at severe conditions, at 600 °C, eliminates bond assignment peaks, revealing reductions in bond deformation. The peak seen at 2849 cm−1, 2850 cm−1, and 2900 cm−1 for raw, T200, and T600, respectively, was generated by the expansion of the structural C-H bond vibrations, which was mostly driven by the aliphatic -CH2- group [67]. Reduced transmittance indicated that the aromatic portions of hemicellulose and cellulose were thermally degrading, and CH2 seemed to have the highest probability of being produced because of the C-H bond breaking [68].
4 Conclusions
The TGA analyses at seven heating rates were carried out for tea-waste mainly in three conditions: raw material sample, torrefied sample at 200 °C, and torrefied sample at 600 °C. The kinetic studies for all cases were performed. The results of the analysis showed high bioenergy potential. The X-ray fluorescence measurement indicated the presence of several mineral compounds in tea-waste, which were influenced by high-temperature thermal treatment. In raw waste-tea, FTIR analysis revealed the presence of moisture, alkene, aromatics, acids, and esters. Various degrees of deformation were caused by their exposure to high-temperature thermal treatment. The results demonstrated that torrefied tea-waste at 600 °C case has a higher heating value (28800 kJ/kg) than raw case (18716 kJ/kg), which is consistent with results reported for other kinds of biomass. Moreover, ΔH was increased gradually with the conversion degree for FWO method, which is 205.01 kJ/mol for hemicellulose, 224.18 kJ/mol for cellulose, and 306.65 kJ/mol for lignin. The results are consistent with the fact that lignin requires the most significant heat energy to dissolve chemical bonds, followed by cellulose and hemicellulose. The determined activation energy aligned well with that published in the scientific literature for other feedstocks. The heating rate combination affected the activation energy values using FWO and KAS methods. Regarding the model-free methods, the minimum and the maximum activation energy values were approximately 80 ± 3 kJ/mol and 415 ± 5 kJ/mol, respectively. The thermodynamic results indicate that tea-waste has a crystalline structure and a high reactivity throughout torrefaction.
Abbreviations
- k :
-
Reaction rate constant [min−1]
- m i :
-
Initial mass [mg]
- m t :
-
Actual mass [mg]
- m f :
-
Final mass [mg]
- n :
-
Reaction order [1]
- α :
-
Degree of conversion
- β :
-
Heating rate [°C min−1]
- A :
-
Pre-exponential factor [min−1]
- DTG:
-
Differential thermogravimetry
- EY:
-
Energy yield
- EF:
-
Enhancement Factor
- E a :
-
Activation energy [kJ mol−1]
- FWO:
-
Flynn-Wall-Ozawa
- KAS:
-
Kissinger-Akahiraa-Sunose
- MY:
-
Mass yield
- R :
-
Gas constant [kJ mol−1K−1]
- R 2 :
-
Correlation coefficient
- TGA:
-
Thermogravimetry analysis
- T200:
-
Torrefied at 200 °C
- T600:
-
Torrefied at 200 °C
- T :
-
Temperature [K]
- XRF:
-
X-ray fluorescence
References
Kim SJ, Um BH (2020) Effect of thermochemically fractionation before hydrothermal liquefaction of herbaceous biomass on biocrude characteristics. Renew Energy 160:612–622. https://doi.org/10.1016/j.renene.2020.07.002
Ikegwu UM, Okoro NM, Ozonoh M, Daramola MO (2022) Thermogravimetric properties and degradation kinetics of biomass during its thermochemical conversion process. Mater Today Proc 65:2163–2171. https://doi.org/10.1016/j.matpr.2022.05.538
Cardoso J, Silva V, Eusébio D, Brito P, Tarelho L (2018) Improved numerical approaches to predict hydrodynamics in a pilot-scale bubbling fluidized bed biomass reactor: a numerical study with experimental validation. Energy Convers Manag 156:53–67. https://doi.org/10.1016/j.enconman.2017.11.005
Gajera B, Tyagi U, Sarma AK, Jha MK (2022) Impact of torrefaction on thermal behavior of wheat straw and groundnut stalk biomass: kinetic and thermodynamic study. Fuel Communications 12:100073. https://doi.org/10.1016/j.jfueco.2022.100073
Duranay ND, Akkuş G (2021) Solid fuel production with torrefaction from vineyard pruning waste. Biomass Convers Biorefin 11:2335. https://doi.org/10.1007/s13399-019-00496-0/Published
Santos DBP, de Jesus MF, Júnior JMF, de Moraes Pires CA (2022) Determination of kinetic parameters for the sisal residue pyrolysis through thermal analysis. J Ind Eng Chem 109:296–305. https://doi.org/10.1016/j.jiec.2022.02.013
Wang C, Zhang L, Chang Y, Pang M (2021) Energy return on investment (EROI) of biomass conversion systems in China: meta-analysis focused on system boundary unification. Renew Sustain Energy Rev 137:110652. https://doi.org/10.1016/j.rser.2020.110652
Amer M, Brachi P, Ruoppolo G, El-Sharkawy I, Ahmed M, Ookawara S, Elwardany A (2021) Pyrolysis and combustion kinetics of thermally treated globe artichoke leaves. Energy Convers Manag 246:114656. https://doi.org/10.1016/j.enconman.2021.114656
Baath YS, Nikrityuk PA, Gupta R (2022) Experimental and numerical verifications of biochar gasification kinetics using TGA. Renew Energy 185:717–733. https://doi.org/10.1016/j.renene.2021.12.091
Wang K, Hu L, Deng J, Zhang Y (2023) Multiscale thermal behavioral characterization of spontaneous combustion of pre-oxidized coal with different air exposure time. Energy 262:125397. https://doi.org/10.1016/j.energy.2022.125397
Habibi Y, El-Zawawy WK, Ibrahim MM, Dufresne A (2008) Processing and characterization of reinforced polyethylene composites made with lignocellulosic fibers from Egyptian agro-industrial residues. Compos Sci Technol 68:1877–1885. https://doi.org/10.1016/j.compscitech.2008.01.008
Phuakpunk K, Chalermsinsuwan B, Assabumrungrat S (2022) Pyrolysis kinetic parameters investigation of single and tri-component biomass: models fitting via comparative model-free methods. Renew Energy 182:494–507. https://doi.org/10.1016/j.renene.2021.10.011
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC kinetics committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19. https://doi.org/10.1016/j.tca.2011.03.034
Zhao R, Liu L, Bi Y, Tian L, Wang X (2020) Determination of pyrolysis characteristics and thermo-kinetics to assess the bioenergy potential of Phragmites communis. Energy Convers Manag 207:112510. https://doi.org/10.1016/j.enconman.2020.112510
Huang S, Lei C, Qin J, Yi C, Chen T, Yao L, Li B, Wen Y, Zhou Z, Xia M (2022) Properties, kinetics and pyrolysis products distribution of oxidative torrefied camellia shell in different oxygen concentration. Energy 251:123941. https://doi.org/10.1016/j.energy.2022.123941
Mohammed HI, Garba K, Ahmed SI, Abubakar LG (2022) Thermodynamics and kinetics of Doum (Hyphaene thebaica) shell using thermogravimetric analysis: a study on pyrolysis pathway to produce bioenergy. Renew Energy 200:1275–1285. https://doi.org/10.1016/j.renene.2022.10.042
Cai H, Liu J, Kuo J, Buyukada M, Evrendilek F (2019) Thermal characteristics, kinetics, gas emissions and thermodynamic simulations of (co-)combustions of textile dyeing sludge and waste tea. J Clean Prod 239:118113. https://doi.org/10.1016/j.jclepro.2019.118113
Prabhakaran SS, Swaminathan G, Joshi VV (2022) Combustion and pyrolysis kinetics of Australian lignite coal and validation by artificial neural networks. Energy 242:122949. https://doi.org/10.1016/j.energy.2021.122949
Parthasarathy P, Fernandez A, Al-Ansari T, Mackey HR, Rodriguez R, McKay G (2021) Thermal degradation characteristics and gasification kinetics of camel manure using thermogravimetric analysis. J Environ Manage 287:112345. https://doi.org/10.1016/j.jenvman.2021.112345
Mohd Safaai NS, Pang S (2021) Pyrolysis kinetics of chemically treated and torrefied radiata pine identified through thermogravimetric analysis. Renew Energy 175:200–213. https://doi.org/10.1016/j.renene.2021.04.117
Choudhary M, Jain SK, Devnani GL, Sonawane SRS, Singh D (2022) Thermal kinetics and morphological investigation of alkaline treated rice husk biomass. J Ind Chem Soc 99:100444. https://doi.org/10.1016/j.jics.2022.100444
Karaeva JV, Timofeeva SS, Islamova SI, Gerasimov AV (2022) Pyrolysis kinetics of new bioenergy feedstock from anaerobic digestate of agro-waste by thermogravimetric analysis. J Environ Chem Eng 10:107850. https://doi.org/10.1016/j.jece.2022.107850
Torres-Sciancalepore R, Fernandez A, Asensio D, Riveros M, Fabani MP, Fouga G, Rodriguez R, Mazza G (2022) Kinetic and thermodynamic comparative study of quince bio-waste slow pyrolysis before and after sustainable recovery of pectin compounds. Energy Convers Manag 252:115076. https://doi.org/10.1016/j.enconman.2021.115076
Torres-Sciancalepore R, Asensio D, Nassini D, Fernandez A, Rodriguez R, Fouga G, Mazza G (2022) Assessment of the behavior of Rosa rubiginosa seed waste during slow pyrolysis process towards complete recovery: kinetic modeling and product analysis. Energy Convers Manag 272:116340. https://doi.org/10.1016/j.enconman.2022.116340
Al-Rumaihi A, Parthasarathy P, Fernandez A, Al-Ansari T, Mackey HR, Rodriguez R, Mazza G, McKay G (2021) Thermal degradation characteristics and kinetic study of camel manure pyrolysis. J Environ Chem Eng 9:106071. https://doi.org/10.1016/j.jece.2021.106071
van de Velden M, Baeyens J, Boukis I (2008) Modeling CFB biomass pyrolysis reactors. Biomass Bioenergy 32:128–139. https://doi.org/10.1016/j.biombioe.2007.08.001
Deng S, Tan H, Wang X, Yang F, Cao R, Wang Z, Ruan R (2017) Investigation on the fast co-pyrolysis of sewage sludge with biomass and the combustion reactivity of residual char. Bioresour Technol 239:302–310. https://doi.org/10.1016/j.biortech.2017.04.067
Paniagua S, Reyes S, Lima F, Pilipenko N, Calvo LF (2021) Combustion of avocado crop residues: effect of crop variety and nature of nutrients. Fuel 291:119660. https://doi.org/10.1016/j.fuel.2020.119660
Chen D, Gao A, Cen K, Zhang J, Cao X, Ma Z (2018) Investigation of biomass torrefaction based on three major components: hemicellulose, cellulose, and lignin. Energy Convers Manag 169:228–237. https://doi.org/10.1016/j.enconman.2018.05.063
Vyazovkin S, Burnham AK, Favergeon L, Koga N, Moukhina E, Pérez-Maqueda LA, Sbirrazzuoli N (2020) ICTAC kinetics committee recommendations for analysis of multi-step kinetics. Thermochim Acta 689:178597. https://doi.org/10.1016/j.tca.2020.178597
Rueda-Ordóñez YJ, Arias-Hernández CJ, Manrique-Pinto JF, Gauthier-Maradei P, Bizzo WA (2019) Assessment of the thermal decomposition kinetics of empty fruit bunch, kernel shell and their blend. Bioresour Technol 292:121923. https://doi.org/10.1016/j.biortech.2019.121923
Siddiqi H, Bal M, Kumari U, Meikap BC (2020) In-depth physiochemical characterization and detailed thermo-kinetic study of biomass wastes to analyze its energy potential. Renew Energy 148:756–771. https://doi.org/10.1016/j.renene.2019.10.162
Basu P (2010) Biomass gasification and pyrolysis: practical design and theory. Academic Press. https://doi.org/10.1016/C2009-0-20099-7
Nour M, Amer M, Elwardany A, Attia A, Li X, Nada S (2021) Pyrolysis, kinetics, and structural analyses of agricultural residues in Egypt: For future assessment of their energy potential. Clean Eng Technol 2:100080. https://doi.org/10.1016/j.clet.2021.100080
Nawaz A, Kumar P (2022) Pyrolysis behavior of low value biomass (Sesbania bispinosa) to elucidate its bioenergy potential: Kinetic, thermodynamic and prediction modelling using artificial neural network. Renew Energy 200:257–270. https://doi.org/10.1016/j.renene.2022.09.110
Amer M, Nour M, Ahmed M, El-Sharkawy I, Ookawara S, Nada SA, Elwardany A (2021) Kinetics and physical analyses for pyrolyzed Egyptian agricultural and woody biomasses: effect of microwave drying. Biomass Convers Biorefin 11:2855. https://doi.org/10.1007/s13399-020-00684-3
Ashraf A, Sattar H, Munir S (2019) A comparative applicability study of model-fitting and model-free kinetic analysis approaches to non-isothermal pyrolysis of coal and agricultural residues. Fuel 240:326–333. https://doi.org/10.1016/j.fuel.2018.11.149
Ong HC, Yu KL, Chen WH, Pillejera MK, Bi X, Tran KQ, Pétrissans A, Pétrissans M (2021) Variation of lignocellulosic biomass structure from torrefaction: a critical review. Renew Sustain Energy Rev 152:111698. https://doi.org/10.1016/j.rser.2021.111698
Huang YF, Chen WR, Chiueh PT, Kuan WH, Lo SL (2012) Microwave torrefaction of rice straw and pennisetum. Bioresour Technol 123:1–7. https://doi.org/10.1016/j.biortech.2012.08.006
Huang X, Cao JP, Zhao XY, Wang JX, Fan X, Zhao YP, Wei XY (2016) Pyrolysis kinetics of soybean straw using thermogravimetric analysis. Fuel 169:93–98. https://doi.org/10.1016/j.fuel.2015.12.011
Raza M, Abu-Jdayil B, Al-Marzouqi AH, Inayat A (2022) Kinetic and thermodynamic analyses of date palm surface fibers pyrolysis using Coats-Redfern method, Renew. Energy 183:67–77. https://doi.org/10.1016/j.renene.2021.10.065
Abdelouahed L, Leveneur S, Vernieres-Hassimi L, Balland L, Taouk B (2017) Comparative investigation for the determination of kinetic parameters for biomass pyrolysis by thermogravimetric analysis. J Therm Anal Calorim 129:1201–1213. https://doi.org/10.1007/s10973-017-6212-9
Ozawa T (1965) A new method of analyzing thermogravimetric data. Chem. Soc. Jpn 38:1881–1886
J.H. Flynn, L.A. Wall, General treatment of the thermogravimetry of polymers, n.d.
Masawat N, Atong D, Sricharoenchaikul V (2019) Thermo-kinetics and product analysis of the catalytic pyrolysis of Pongamia residual cake. J Environ Manage 242:238–245. https://doi.org/10.1016/j.jenvman.2019.04.080
Liang YG, Cheng B, Si YB, Cao DJ, Jiang HY, Han GM, Liu XH (2014) Thermal decomposition kinetics and characteristics of Spartina alterniflora via thermogravimetric analysis. Renew Energy 68:111–117. https://doi.org/10.1016/j.renene.2014.01.041
Slopiecka K, Bartocci P, Fantozzi F (2012) Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl Energy 97:491–497. https://doi.org/10.1016/j.apenergy.2011.12.056
Vyazovkin S (2020) Kissinger method in kinetics of materials: things to beware and be aware of. Molecules 25:2813. https://doi.org/10.3390/molecules25122813
Kissnger H (1956) Variation of peak temperature with heating rate in differential thermal analysis. J Res Natl Bur Stand 57:217–221
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706. https://doi.org/10.1021/ac60131a045
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201:68–69. https://doi.org/10.1038/201068a0
Mishra RK, Mohanty K, Wang X (2020) Pyrolysis kinetic behavior and Py-GC–MS analysis of waste dahlia flowers into renewable fuel and value-added chemicals. Fuel 260:116338. https://doi.org/10.1016/j.fuel.2019.116338
Yuan X, He T, Cao H, Yuan Q (2017) Cattle manure pyrolysis process: kinetic and thermodynamic analysis with isoconversional methods. Renew Energy 107:489–496. https://doi.org/10.1016/j.renene.2017.02.026
Chen D, Chen F, Cen K, Cao X, Zhang J, Zhou J (2020) Upgrading rice husk via oxidative torrefaction: characterization of solid, liquid, gaseous products and a comparison with non-oxidative torrefaction. Fuel 275:117936. https://doi.org/10.1016/j.fuel.2020.117936
M. Amer, A. Elwardany, Biomass carbonization, in: 2020. https://doi.org/10.5772/intechopen.90480.
Cantrell KB, Hunt PG, Uchimiya M, Novak JM, Ro KS (2012) Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour Technol 107:419–428. https://doi.org/10.1016/j.biortech.2011.11.084
Annamalai K, Puri IK (2006) Combustion science and engineering. CRC Press
El-Sayed SA, Mostafa ME, Kinetics, thermodynamics, and combustion characteristics of Poinciana pods using TG/DTG/DTA techniques., Biomass Convers Biorefin (2021). https://doi.org/10.1007/s13399-021-02021-8.
Han J, Yu D, Wu J, Yu X, Liu F, Xu M (2023) Effects of torrefaction on ash-related issues during biomass combustion and co-combustion with coal. Part 2: Ash fouling behavior. Fuel 334:126777. https://doi.org/10.1016/j.fuel.2022.126777
El-Sayed S, Mostafa M (2014) Pyrolysis characteristics and kinetic parameters determination of biomass fuel powders by differential thermal gravimetric analysis (TGA/DTG). Energy Convers Manag 85:165–172. https://doi.org/10.1016/j.enconman.2014.05.068
Sarker TR, Azargohar R, Dalai AK, Meda V (2021) Enhancement of fuel and physicochemical properties of canola residues via microwave torrefaction. Energy Rep 7:6338–6353. https://doi.org/10.1016/j.egyr.2021.09.068
Chen D, Zheng Z, Fu K, Zeng Z, Wang J, Lu M (2015) Torrefaction of biomass stalk and its effect on the yield and quality of pyrolysis products. Fuel 159:27–32. https://doi.org/10.1016/j.fuel.2015.06.078
Mishra RK, Mohanty K (2018) Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis. Bioresour Technol 251:63–74. https://doi.org/10.1016/j.biortech.2017.12.029
Heydari M, Rahman M, Gupta R (2015) Kinetic study and thermal decomposition behavior of lignite coal. Int J Chem Eng 2015:481739. https://doi.org/10.1155/2015/481739
Khan AS, Man Z, Bustam MA, Kait CF, Ullah Z, Nasrullah A, Khan MI, Gonfa G, Ahmad P, Muhammad N (2016) Kinetics and thermodynamic parameters of ionic liquid pretreated rubber wood biomass. J Mol Liq 223:754–762. https://doi.org/10.1016/j.molliq.2016.09.012
Mehmood MA, Ye G, Luo H, Liu C, Malik S, Afzal I, Xu J, Ahmad MS (2017) Pyrolysis and kinetic analyses of camel grass (Cymbopogon schoenanthus) for bioenergy. Bioresour Technol 228:18–24. https://doi.org/10.1016/j.biortech.2016.12.096
Li MF, Li X, Bian J, Chen CZ, Yu YT, Sun RC (2015) Effect of temperature and holding time on bamboo torrefaction. Biomass Bioenergy 83:366–372. https://doi.org/10.1016/j.biombioe.2015.10.016
Ma Z, Wang J, Yang Y, Zhang Y, Zhao C, Yu Y, Wang S (2018) Comparison of the thermal degradation behaviors and kinetics of palm oil waste under nitrogen and air atmosphere in TGA-FTIR with a complementary use of model-free and model-fitting approaches. J Anal Appl Pyrolysis 134:12–24. https://doi.org/10.1016/j.jaap.2018.04.002
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alashmawy, M.M., Hassan, H.S., Ookawara, S.A. et al. Thermal decomposition characteristics and study of the reaction kinetics of tea-waste. Biomass Conv. Bioref. 13, 9487–9505 (2023). https://doi.org/10.1007/s13399-023-04017-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-023-04017-y