Abstract
The replacement of expensive components in microbial growth media with pretreated lignocellulosic waste component to increase the product spectrum and add value to the bioproducts has been encouraged to achieve sustainable and feasible utilization of waste biomass as per the biorefinery approach. This study demonstrates an integrated biorefinery approach towards utilization of sugarcane bagasse and biomass of Mucor circinelloides ZSKP. A maximum reducing sugar recovery of 80.67 g/l was achieved after combining pretreatment with saccharification. A low temperature, glycerol, and ammonium phosphate pretreatment method was established, where glycerol pretreatment conditions were reduced from 250 to 150 °C and from 120 to 45 min. The ammonium phosphate-containing hydrolysate yielded 12.89 g/l of fungal biomass after fermentation to add to 20.8 g lignin from the delignification step. The biomass production was further improved to 17.69 g/l after supplementation with corn steep solids and mineral salts. The fermentation process also yielded 2.36 g/l chitosan and 4.9 g/l of lipids after extraction from the oleaginous fungus. The lignin infused glycerol plasticized chitosan biocomposite plastic had a 100% improvement in thermogravimetric properties with almost 50% more energy needed to increase the temperature of the material when compared to glycerol only plasticized biocomposite. The fungal chitosan showed antimicrobial properties and was effective as a preservative spray for fresh tomatoes and apples extending their shelf life to at least 14 and 18 days, respectively. This study therefore demonstrated that a novel two-step pretreatment process could be environmentally beneficial and yielded multiple products for biotechnological applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to the International Energy Agency (IEA) Bioenergy Task 42, a biorefinery is the sustainable processing of biomass into a spectrum of marketable products and energy [1, 2]. The global biorefinery market is expected to reach USD 52,680 million by 2027, at a compound annual growth rate of 2.2% [3]. The efficiency of these biorefineries can be enhanced by producing value-added co-products during the process [4,5,6]. Sustainable and economical bioconversion process is a prerequisite for the biorefinery concept. Sugarcane-based biorefineries have been a leading approach in the production of cellulosic biofuels.
With an annual production of about 25 million tons, South Africa is the largest producer of sugarcane in Africa. However, the country also produces about 7.5 million tons of bagasse annually during sugarcane processing, which largely remains underutilized [7, 8]. Therefore, efficient utilization of this waste biomass becomes expedient. This can be achieved by incorporating processing steps for the wastes generated after various conversion steps. The utilization of biomass such as bagasse, which contains complex polymers like cellulose, hemicellulose, and lignin, is performed by a combination of physico-chemical pretreatment methods and enzymatic breakdown of the free cellulose and hemicellulose after pretreatment [9, 10]. Despite active research on the use of sugarcane bagasse (SCB) to bioethanol and other biochemicals, low saccharification due to recalcitrance of biomass remains a global challenge.
Pretreatment is a crucial step in biorefinery process that facilitates enzymatic access to the trapped cellulose within the crystalline structure of lignocellulosic and agro-based residue like SCB, for subsequent hydrolysis. Pretreatment enhances the access of saccharification enzymes to the carbon polymers. During the last few years, various chemical, physical, and biological pretreatment methods have been developed to expand the surface area, improve the overall porosity, dissolve the hemicellulose and lignin, and reduce the particle size of the recalcitrant lignocellulosic biomass [11]. However, there is an immense variation in composition and structure of lignocellulosic biomass, and therefore, no single universal pretreatment method can be applied to all types of lignocellulosic biomass [12]. There is a need to combine the pretreatment processes to achieve a balanced strategy with higher efficacy. Additionally, removal of sturdy lignin, which is highly resistant to solubilization, is a major technical and economic challenge [13].
Although dilute acid pretreatment is most commonly used, but due to several disadvantages associated with this method, alkali pretreatment emerged as a promising alternative. An attractive feature of this method is its ability to remove lignin with selective retention of cellulose. This mode of action shares some similarities with the organosolv pretreatment. However, the organosolv process is targeted towards delignification with solubilization of hemicellulose to varying degrees. Glycerol has been reported to be effective as an organosolv. The use of glycerol raises interesting prospects since it is a byproduct of the biodiesel production process [14, 15]. The combination of alkaline and organosolv pretreatment methods may result in lignin extraction under milder processing conditions. High energy costs and high costs of robust infrastructure to withstand extreme pretreatment conditions are always deleterious to biorefinery projects. The combination of pretreatment methods may result in the amelioration of harsh and expensive pretreatment conditions without compromising on the desired yield. An ideal pretreatment method should be cost-effective, minimize the release of inhibitors, minimize water consumption, and safe to the environment without compromising the yield of fermentable sugars.
Previously, Mucor circinelloides ZSKP was used for concurrent production of chitosan and lipids, with the lipids being used for biodiesel production [16]. The oleaginous zygomycete, M. circinelloides, is of special interest due to its ability to produce lipids, chitosan, pigments, ethanol, and many other industrially important biomolecules [17]. Interestingly, this dimorphic fungus is reported to grow on several lignocellulosic biomass including SCB [18], corn straw [19], wheat straw, pomegranate, and tangerine peels [20]. This study was focused on developing a biorefinery approach by valorization of M. circinelloides ZSKP and SCB biomass through a new pretreatment strategy using alkalic salts and glycerol to generate supplemented bagasse hydrolysate as an alternative cultivation medium. An attempt was made to implement a multi-step valorization approach to produce a wide array of bio-products. Additionally, produced lignin and chitosan were utilized for value-addition.
2 Materials and method
2.1 Fungal cultivation
Mucor circinelloides ZSKP, previously isolated by Zininga et al. [16], was sub-cultured on potato dextrose agar plates at 27 °C for 72 h. Spores of M. circinelloides ZSKP were collected from agar plates using sterile distilled water and used as seed cultures for fermentation. The strain was cryopreserved in 15% (v/v) glycerol stock solution at − 80 °C.
2.2 Composition of bagasse
SCB, obtained from a local sugar mill, was washed and air-dried. Dry SCB was milled into fine biomass and sieved into a storage container using a 300-µm sieve. Cellulose (44.12%), hemicellulose (23.89%), and lignin (25.78%) were quantified according to methods described by Sluiter et al. [21].
2.3 Pretreatment of SCB
Five grams of bagasse was initially pretreated using 50 ml of 1% sulfuric acid, 1.5% sodium hydroxide, and 80% glycerol at 120 °C, 160 °C, and 250 °C, respectively, at durations of 1 h for the sulfuric acid and sodium hydroxide pretreatments and 2 h for the glycerol pretreatment. A combined pretreatment and lignin extraction (CPTL) was used to combine the glycerol (80%, 120 °C, 15 min) and dilute alkaline sodium hydroxide (1.5%, 120 °C, 15 min) pretreatment methods. The alkaline sodium hydroxide was replaced by 5% alkalic salts such as sodium phosphate, sodium carbonate, ammonium phosphate, and ammonium carbonate [22]. The combination eventually included initial glycerol pretreatment and first lignin extraction step (L1) with a 1.5% sodium hydroxide lignin extraction (L2) being the final recovery step after saccharification as per a modified combined pretreatment and lignin extraction (mCPTL) strategy. The pretreatment was done in 1 l Schott bottles which were sealed and immersed in an oil bath (Schutzart DIN 40,050-1P20) preheated to the desired temperature. The effect of different pretreatment parameters such as different temperatures of the glycerol pretreatment, different pretreatment duration times, and for the ammonium phosphate pretreatment such as different temperatures and concentrations on total reducing sugar yield was investigated in triplicate. The mean difference between different pretreatments was analyzed by ANOVA.
2.4 Saccharification
Three commercial enzyme cocktails containing cellulase (500,000 U/g), xylanase (290,000 U/g), and β-glucosidase (50,000 U/g) purchased from Shandong Longda Bio-Products Company Limited (China) were loaded at a ratio of 10 mg of enzyme per gram of dried pretreated bagasse substrate as reported by Osipov et al. [23]. The enzyme cocktail was dissolved in 50 ml acetate buffer (0.05 M, pH 5.5). The saccharification was carried out in a 250-ml Erlenmeyer flask for 48 h at 50 °C. The final sugar concentrations were analyzed using high-performance liquid chromatography (HPLC) and 3,5-dinitrosalicyclic acid method. The reducing sugars yield was calculated using the formulae reported by Qing et al. [22].
2.5 HPLC analysis
Concentration of different sugars in liquid samples after enzymatic hydrolysis was analyzed by HPLC (Shimadzu LCMS-2020). The HPLC system was equipped with an ELSD detector and Aminex HPX-87P column. Degassed Milli-Q water was used as the mobile phase at a flow rate of 0.6 ml/min at 50 °C. Analytical standards of sugars (Sigma Aldrich) were used for determination of unknown concentrations.
2.6 Supplemented bagasse hydrolysate as a medium for fungal growth
The hydrolysate obtained after saccharification was supplemented with 5% corn steep solids and inoculated with M. circinelloides spores in 250-mL Erlenmeyer flasks containing 50 mL medium (pH 5.5) at 250 rpm, 30 °C for 120 h. A 180-µm sieve and filter cloth were used to separate the media and biomass. The biomass was freeze-dried to obtain the dry biomass weight.
2.7 Lignin, lipids, and chitosan extraction
Lignin was initially extracted using different concentrations of commercial glycerol (Sigma) and 1.5% sodium hydroxide at 120 °C, with both extractions being carried out just before saccharification. The best lignin recovery (80%) was eventually achieved by pretreatment at 150 °C, followed by saccharification and then a 1.5% sodium hydroxide extraction being implemented at 120 °C on the residue after saccharification. The lignin residue was precipitated from the supernatant by adjusting the pH to 2.0 using 1 M HCl and oven drying at 60 °C. The lipids and chitosan were concomitantly extracted according to Zininga et al. [16].
2.8 Preparation of chitosan biocomposite films
A 1% (w/v) chitosan sample in 1% acetic acid was prepared with constant stirring at 23 °C. This was kept overnight with constant agitation. Thereafter, particulates were removed by centrifugation. Glycerol and glycerol with dissolved lignin (from the lignin extraction process) were added as plasticizers. Each plasticizer was added to attain a final plasticizer to chitosan weight ratio of 0.04 [24]. The emulsions were then poured into petri plates after removal of bubbles and dried in an oven (35 °C, 24 h). The casted plastic films were recovered by peeling off the plate.
2.9 Thermogravimetric analysis
Thermogravimetric analysis was used to evaluate the degradation of the chitosan bioplastic with glycerol and lignin-infused glycerol plasticizers. An SDT Q600 V20.9 Build 20 thermal analyzer (TA instruments) was used over a temperature range of 25–590 °C at 10 °C/min heating rate. The tested samples weighed 5 and 10 mg each. Nitrogen was used over the samples. The weight loss was assessed using the associated software and expressed as a percentage [25].
2.10 FTIR analysis
The samples of extracted chitosan were characterized by FTIR spectroscopy (Cary 630, Agilent) in the range of 400 to 4000 cm−1. The transmission spectra of acetic and sulfuric acid-extracted chitosan were then compared to the spectrum produced by commercial chitosan extracted from shrimp shells (Sigma).
2.11 Determination of MLC for fungal chitosan
One gram of fungal chitosan was dissolved in 1% acetic acid to make up 1% (m/v) chitosan solution. The chitosan solution was further diluted using 0.9% physiological saline to make diluted concentrations of 0.1%, 0.08%, 0.06%, 0.04%, and 0.02% chitosan solution (w/v). The pH for all the solutions was adjusted to 5.5 using 2 M sodium hydroxide. After autoclaving, 10 ml chitosan solutions were inoculated with 0.05 ml of freshly prepared microbial cultures Escherichia coli, Staphylococcus aureus, Bacillus subtilis, and Aspergillus niger followed by mixing. The cultures were incubated with different concentrations of chitosan solution at 37 °C for 24 h. Viable cells were enumerated by spread plating on nutrient agar plates (for bacterial) and potato dextrose agar (for fungi) then incubating at 37 °C for 48 h. The minimum lethal concentration was defined as the minimum concentration of chitosan that kills all microbial cells. Two controls were used: 1% acetic acid only and with water.
2.12 Application of a fungal chitosan spray for fresh fruit produce
Apples and tomatoes, purchased from a local market, were used for testing the antimicrobial applicability of the extracted fungal chitosan. Apples and tomatoes were first washed with tap water, then with distilled water, and finally sprayed with 70% alcohol and left to dry naturally at ambient temperature. Three groups of fruits were involved in each experiment. The first one involved chitosan 0.5% (m/v) dissolved in 1% acetic acid which was sprayed onto the fresh fruits. The control group involved using a 1% acetic acid spray without the chitosan. The second control group consisted of the fruits sprayed only with water. The presence of spoilage was then observed.
3 Results and discussion
3.1 Pretreatment methods
3.1.1 Comparison of pretreatment methods
The effect of sulfuric acid, glycerol, and sodium hydroxide pretreatment of bagasse on reducing sugar yield was studied after enzymatic saccharification. SCB pretreated with sodium hydroxide resulted in 89.9 g/l of reducing sugars. The lowest amount of reducing sugar was released when pretreated with sulfuric acid (34.6 g/l), while glycerol pretreatment resulted in 79.4 g/l reducing sugars.
Pretreatment is the most important step in the valorization of agro-based residue like SCB. It is meant to enhance access of the saccharification enzymes to the carbon polymers and not necessarily to release fermentable sugars. It has been noted in literature that both alkaline (sodium hydroxide) and dilute acid (sulfuric acid) pretreatment methods release fermentation and saccharification inhibitors with the resultant hydrolysates needing detoxification steps. There is need to balance between a higher effective pretreatment and a lower release of saccharification and fermentation inhibitors. This brings about the possibility of combining the pretreatment processes to strike a balance between a high yield of fermentable sugars after saccharification and a low or no release of inhibitors. Other factors such as high energy costs and high costs of robust infrastructure to withstand extreme pretreatment condition are always considered as well; hence, the combination should result in the moderation of the harsh and expensive pretreatment conditions without compromising on the desired yield.
Acid pretreatment is not efficient in dissolving lignin, which acts as a barrier to impede saccharification [26]. This may be the reason for the lower reducing sugars (34.6 g/l) from the sulfuric acid method when compared to the sodium hydroxide and glycerol pretreatment methods. The current pretreatment strategies for SCB have focused on delignification, hence, the current trend towards mostly alkaline pretreatment methods and, in some instances, organosolv pretreatment methods. Alkaline pretreatment also cleaves the uronic acid and acetyl groups on hemicellulose. This has an overall positive reactivity effect on the polysaccharides [27], while the organosolv pretreatment partially breaks down lignin bonds very efficiently. This results in a pulp that is rich in cellulose as it will also solubilize most of the hemicellulose sugars. The lignin selectivity of the solvent can be improved by adding a catalyst [28, 29]. Unlike the aqueous process of alkaline delignification, the organosolv pretreatment offers the advantages of easier lignin recovery and concurrent recycling of the solvent through distillation [29, 30].
3.2 CPTL
Lignin was concomitantly extracted during pretreatment using a combination of different strategies (L1 and L2; Table 1). Using both L1 and L2 strategies, maximum amount of total lignin was produced (0.4 g and 0.65 g, respectively) using 80:20 (80% glycerol) glycerol to water ratio (Table 1). However, lowest amount of reducing sugars (69.38 g/l) was produced using 80% glycerol. The pretreatment, in which 1.5% sodium hydroxide was used without glycerol, showed highest reducing sugar (90.6 g/l), but with lowest amount of total lignin.
The lignin yields obtained are consistent with Novo et al. [30], who used a mixture of glycerol and water resulting in a pulp with less than 8% residual lignin. At least 80% delignification was achieved. However, the conditions that gave the highest lignin extraction yield did not correspond to the highest yield of reducing sugars, but rather a low yield of 69.3 g/l. This suggests that there was release of saccharification inhibitors. This is exacerbated by the combination of high temperatures as well as the high degree of alkaline lignin solubilization, which potentially releases chemicals like furans and phenolic compounds [31, 32].
Glycerol pretreatment as an organosolv process can be used for treatment of lignocellulosic materials like bagasse. The lignin was recovered easily with a possibility of recycling the solvents used. It is, however, important to note the comparatively milder conditions employed in this study. High temperature of 250 °C needed to apply the glycerol pretreatment means high energy costs and the need for costly infrastructure. Combination of solvents like glycerol and sodium hydroxide can prevent reprecipitation of the dissolved lignin, thereby enhancing lignin recovery.
3.3 Pretreatment and growth of M. circinelloides ZSKP
3.3.1 Growth of M. circinelloides ZSKP on different hydrolysates
The hydrolysate from the bagasse samples pretreated with sodium hydroxide and glycerol was used as medium for the growth of M. circinelloides ZSKP. The glycerol pretreatment-derived hydrolysate resulted 1.93 g/l of biomass, while no growth was observed in the sodium hydroxide pretreatment-derived hydrolysate. A combination of glycerol and sodium hydroxide pretreatment also yielded no growth.
Sodium hydroxide used in the alkaline pretreatment process breaks ester bonds between the lignin and hemicelluloses due to saponification reaction. This allows enhanced access of saccharifying enzymes to cellulose for subsequent hydrolysis. However, several fermentation inhibitors may be released when the aqueous lignin is broken down at high temperatures. The non-aqueous nature of glycerol lignin extraction [33] seems to reduce the release of fermentation inhibitors like hydroxy methyl furfural (HMF) and phenolic compounds despite the high temperatures. This can be indirectly corroborated by the presence or absence of fungal growth.
3.3.2 Alkalic salt pretreatment methods and growth of M. circinelloides ZSKP
The possibility of an alkaline pretreatment method that is less rigorous like the use of weaker alkali salts as reported by [22] was explored. Sodium phosphate pretreatment gave the highest reducing sugars yield of 38.9 g/l followed by ammonium phosphate at 21.7 g/l. However, both sodium phosphate and ammonium phosphate pretreatments gave reducing sugar yield much lower than sodium hydroxide pretreatment.
Alkaline pretreatment of biomass results in delignification involving condensation and redistribution of lignin, solubilization of the lignin, and changes to cellulose crystalline state. The extent of the lignin targeting is influenced by the type of alkaline solution. The high temperatures applied and a high degree of lignin solubilization increase the chances of fermentation and saccharification inhibitors [34, 35]. A consideration of these factors would explain ammonium phosphate’s potential suitability as a pretreatment solvent that releases minimum amount of inhibitors.
The bagasse hydrolysate from the alkalic salts pretreatment was used as medium for M. circinelloides ZSKP fermentation with biomass yield quantified. There was no growth in the sodium phosphate hydrolysate; however, there was growth on the ammonium phosphate pretreated hydrolysate (1.69 g/l) as well as the combined pretreatment of glycerol and ammonium phosphate (2.56 g/l).
The liquor obtained from the sodium phosphate pretreatment was black, pointing towards a high degree of delignification and solubilization of lignin, unlike the liquor obtained from the ammonium phosphate pretreatment, which was faint brown in color. This suggests that ammonium phosphate, while enhancing accessibility of the cellulose to the enzymes, does not completely solubilize the lignin; hence, lower amounts or no inhibitors were released.
3.4 mCPTL
3.4.1 Combination of glycerol and ammonium phosphate pretreatment methods
The glycerol pretreatment was combined with ammonium phosphate pretreatment. Glycerol pretreatment was maintained at 120 °C, followed by ammonium phosphate pretreatment at a reduced temperature of 80°. The reducing sugars release was improved from 18.9 to 29.8 g/l.
3.4.2 The effect of different parameters on mCPTL
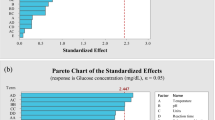
While studying the effect of different temperatures on the release of reducing sugars during ammonium phosphate pretreatment, it was observed that temperatures beyond 70 °C did not resulted a significant increase in the yield (Fig. 1). It should be noted that an initial glycerol pretreatment at 120 °C preceded this step. Therefore, 70 °C was selected as the most viable temperature for this pretreatment. Although maximum release of reducing sugars was observed at 100 °C, it could not be selected for further studies due to increased energy costs.
Increasing the pretreatment temperature for the glycerol lignin extraction resulted in an increase in the reducing sugars yield. The largest increase was observed between 120 and 150 °C, but there was no substantial increase between 150 and 180 °C (Fig. 2a). The second step, ammonium phosphate pretreatment, was performed at 70 °C for 2 h. Therefore, 150 °C was selected due to high yield of reducing sugars and possibly lower energy costs.
The impact of different glycerol pretreatment times on the release of reducing sugars was also studied. After 45 min of pretreatment, the reducing sugars yield increased to 60.96 g/l. Therefore, 45 min was selected as further increases in pretreatment time did not result in a proportional increase in reducing sugars yield, making it potentially economically unfeasible (Fig. 2b).
3.4.3 Concurrent pretreatment and saccharification by mCPTL
Ammonium phosphate pretreatment was combined with saccharification to study its effect on the release of reducing sugars. The combined glycerol-ammonium sulphate pretreatment 6 showed the highest amount of reducing sugars (80.67 g/l) after saccharification (Table 2). The hydrolysate was then used directly as medium for the growth of M. circinelloides ZSKP. This resulted in 12.89 g/l biomass (pretreatment 6; Table 2), which was twice the biomass obtained by using hydrolysate from pretreatment 1.
The ammonium phosphate played a dual role by enhancing the accessibility of cellulose to the saccharification enzymes, as well as source of nitrogen to supplement the hydrolysate during fermentation. This can save costs in establishing a sustainable biorefinery process. This also allows for the moderation of the glycerol pretreatment process to levels that reduces energy costs.
3.5 Replacement of growth medium with bagasse hydrolysate
The bagasse hydrolysate generated from the two-step glycerol and ammonium phosphate pretreatment and saccharification process was used as fermentation medium, and this was supplemented with 5% corn steep solids. A 100-g sample of bagasse gave a lignin yield of 20.8 g and M. circinelloides ZSKP biomass yield of 17.69 g (Table S1). The fungal biomass was used to extract 2.36 g and 4.9 g of chitosan and lipids, respectively.
Lipids produced from M. circinelloides ZSKP were used to produce biodiesel as reported in previous work [20]. Biomass valorization is at the center of addressing feasibility concerns when it comes to the commercialization of biorefineries [36]. Lignin, lipids, and chitosan where successfully produced from the sugar cane bagasse and M. circinelloides biomass (Table S1) with value addition of these products being the next focus.
3.6 Characterization and applications of chitosan and lignin
3.6.1 Characterization of the extracted lignin using FTIR analysis
The lignin extracted from the bagasse was compared to a commercial lignin standard using FTIR analysis. Functional groups associated with lignin including hydroxyl, carboxyl, carbonyl, and methoxy peaks at 3400–3600 cm−1, 2920 cm−1, 1700–1750 cm−1, and 2650–2890 cm−1, respectively, were detected (Fig. S1a).
The band at 1600 cm−1 represented by aromatic skeletal vibrations was clearly visible in both the commercial lignin and the extracted lignin. The picks at 1600 cm−1 from both the extracted and commercial lignin appeared possibly because of the deformation of the hydrogen bonds. Increasing temperature was reported to cleave the intramolecular and intermolecular hydrogen bonds in lignin. The aromatic ring skeleton that conjugates the carbonyl stretch appeared at 1600 cm−1. All specific peaks were present in the spectra of both lignin samples. This evidenced an intact backbone of lignin [37].
3.6.2 Characterization of chitosan by FTIR analysis
FTIR analysis was done for both the acetic acid and sulfuric acid-derived chitosan with commercial shrimp shell chitosan as a reference (Fig. S1b). The peaks at 3417 cm−1 corresponded to the –OH functional group, and the peak at 3417 cm−1 was characteristic of amino group vibrations. The methylene stretching –CH was identified by the presence of the 2877 cm−1 peak. The deformation of the –NH amino group was highlighted by the peak at 1600 cm−1. Peaks at 1631 and 1747 cm−1 represented amide carbonyl and ester carbonyl group stretch vibrations, respectively. The glucose amine ring was shown by the highlighting of the –COC stretch vibrations due to the presence of the 1072 cm−1 peak. All these functional groups confirmed characteristics of chitosan.
3.6.3 The effect of glycerol plasticizer on the tensile properties of the chitosan biocomposite plastic
Additives are necessary in bioplastics to improve the mechanical properties of the plastic and make it more resilient, strong, and soft. Glycerol has been shown to be effective as a plasticizer. Glycerol was used as a plasticizer on its own, as well as with dissolved lignin taken directly from the lignin extraction process. These plasticizers were added to the chitosan bioplastics to create different plasticized samples. The bioplastics with the added plasticizers showed improved tensile strength and thickness (Table 3). However, addition of a plasticizer was shown to negatively affect the thermal properties of the bioplastic (Fig. 3a).
3.6.4 Thermogravimetric and differential scanning calorimetry analysis of the extracted lignin incorporated with chitosan bioplastics
Glycerol containing extracted lignin was directly used as a plasticizer in the chitosan-based biocomposite films. Glycerol with dissolved lignin conferred clear improvements in the thermostability of the plastic having the least biomass loss, retaining almost 50% of its weight when exposed to high temperatures of up to 600 °C (Fig. 3b) as compared to the chitosan biocomposite plastic without plasticizer (Fig. 3c). There was also more heat needed to increase the temperature of the same chitosan biocomposite plastic with lignin-infused plasticizer. The chitosan biocomposite plastic with glycerol-only plasticizer had the most inferior thermal properties, which lost 70% of its weight and required the least amount of heat to increase its temperature. (Fig. 3a).
Plastic has numerous applications, and this can determine which mechanical properties are more desirable. Glycerol has been reported to be a very good plasticizer for biocomposite plastics for polymers such as starch and chitosan. The presence of glycerol in the biocomposite plastic matrix increases elasticity and decreases material resistance as well as the glass transition temperature. However, this comes at the cost of thermal properties of the biocomposite plastic [38, 39]. Glycerol, a polyol, has hydrophilic groups to absorb moisture using hydrogen bond interactions with water molecules. Packaging is one area in which the potential application of chitosan plastic can be pursued. Thermostable properties of plastic are critical in the food industry where heat is applied for preservation and extension of shelf-life. In fact, a lack of thermostability has been one of the major limiting factors to industrial application of plastics [40]. Therefore, plasticized chitosan biocomposite with enhanced thermal properties due to the addition of lignin would open up several new possibilities.
3.6.5 Antimicrobial properties of chitosan
The extracted chitosan showed minimum lethal concentration (MLC) on the four microbial strains from a minimum concentration of 600 to 1100 mg/l, while the commercial shrimp shell chitosan showed activity from 300 to 2000 mg/l (Table S2). For E. coli and S. aureus, the MLC values for fungal chitosan were higher, and for B. subtilis, the MLC values for both were identical. The fungal chitosan was more effective against A. niger with an MLC of 1100 mg/l compared to 2000 mg/l for commercial chitosan.
Previously, biochemical, biological, and molecular analysis have been performed on chitosan to investigate antimicrobial properties. The studies indicated intrinsic and extrinsic factors of chitosan that affect its efficacy as an antimicrobial agent. The extrinsic factors would be environmental conditions and the cellular morphology. The intrinsic chitosan properties include molecular weight, degree of deacetylation (which influences solubility), and complex-ion formation. Polycationic biochemical structure is key in antimicrobial activity. This means the highly intense the positive charge density, the more the electrostatic interaction. Thus, the potential of degree of deacetylation in affecting antimicrobial activity lies within its effect on the positive charge density. Unlike beta-lactam antibiotics, chitosan antibacterial activity goes beyond preventing the synthesis of the peptidoglycan cell wall [41, 42]. One of the major differences between fungal-derived chitosan (LMW) and crustacean shell-derived chitosan (HMW) is molecular weight. There have been conflicting reports on the actual effects of low and high molecular weight chitosan. While some reports showed increased antimicrobial activity with increased molecular weight, a few other reports indicate an increase in antimicrobial activity with decreasing molecular weight. Chitosan interacts with cell-receptor molecules on the cell-wall to form a barrier that prevents nutrient intake. Another mechanism suggests a lower molecular weight chitosan pervasively entering the cell, thereby disrupting the physiological activity of the cell [43]. It seems from the results obtained from this study that while molecular weight of the chitosan may be important for antimicrobial effect, this is also dependent on the nature of the cells targeted. The fungal chitosan was less effective in killing E. coli and S. aureus but had the same lethality on B. subtilis as crustacean chitosan. The fungal chitosan was more effective against A. niger. It is also possible that increasing the purity of the fungal chitosan to the same levels as the commercial chitosan may increase the potency of the fungal chitosan.
3.6.6 Application of fungal chitosan to increase the shelf life of fresh fruit produce
The antimicrobial effects of fungal chitosan against bacterial and fungal strains were studied in a range of 600–1100 mg/l. A chitosan spray was developed using 0.5% (5000 mg/l) chitosan and dissolved in 1% acetic acid, and the spray was used on fresh fruits purchased from a local fresh produce store. Unsprayed fruits and those sprayed with 1% acetic acid were used as controls. The chitosan spray preserved the tomatoes and apples for a longer duration than respective controls. The chitosan sprayed apples and tomatoes were still preserved even after 18 and 14 days of storage at room temperature, respectively, when compared to the controls (Fig. 4).
The effect of fungal chitosan spray on tomatoes and apples; whole tomato a after 14 days without spray, b sprayed with 1% acetic acid, and c sprayed with 1% chitosan; cut tomatoes d after 4 days without spray, e sprayed with 1% acetic acid, and f sprayed with 1% chitosan; apples g after 18 days without any spray, h sprayed with 1% acetic acid, and i sprayed with 1% chitosan
The tomatoes showed evidence of fungal contamination and decay around the areas where the pedicels attach. After 14 days, chitosan protected tomato still retained the overall shape (Fig. 4a–c). However, slight dehydration was noticed on all three fruits. The cut tomatoes showed severe infection by fungi and bacteria after 4 days (Fig. 4d). Interestingly, cut tomatoes sprayed with chitosan were largely free of microbial growth (Fig. 4f). As expected, drying and size reduction of tomatoes were also observed. There was evidence of microbial rot on tomatoes (Fig. 4e) and apples (Fig. 4h) sprayed with 1% acetic acid after 14 and 18 days, respectively. In the same period, apples sprayed with 1% chitosan appeared normal and there were no signs of infection (Fig. 4i). The size and shape looked normal and visually appeared to be fine for consumption.
Fungal chitosan can play a critical role in addressing the challenge of fresh produce being lost to spoilage when incorporated in rapid post-harvest surface sanitation [44]. The fungal chitosan has an advantage when making a chitosan spray for surface sanitation, because of its lower viscosity. This permits higher concentrations of the chitosan for application. It is difficult to make a chitosan spray from crustacean chitosan especially at concentrations beyond 0.5% due to the high viscosity. Fungal chitosan does not have allergenic proteins that is generally prevalent in crustacean-derived chitosan, thus enhancing its suitability in food preservation [45]. The chitosan spray was also applied on cut tomatoes to improve shelf-life. In tomato production, an exposed or cut fruit can be the source of spoilage for the entire batch. Methods that enhance the shelf-life of cut fruits are currently being encouraged due to increasing demand in the global market. Chitosan spray is potentially important to prolong the shelf-life of fresh fruit produce.
4 Conclusions
An integrated process of lower temperature glycerol-ammonium phosphate lignin extraction and bagasse pretreatment combined with saccharification was established. The process resulted a relatively high yield of total reducing sugars, which can be used for microbial growth and production of many other industrially important biomolecules. Ammonium phosphate was proved to be effective both in pretreatment and in supplementing the hydrolysate as a possible source of nitrogen. Addition of lignin to the product spectrum and its application together with chitosan add value and further enhance implementation of the biorefinery concept.
Data availability
Data and materials will be available on request.
References
de Jong E, Higson A, Walsh P, Wellisch M (2012) Bio-based chemicals value added products from biorefineries. IEA Bioenergy, Task 42 Biorefinery 34:1–33
Cheng Y, Wu Z, Sriariyanun M (2019) Evaluation of Macaranga tanarius as a biomass feedstock for fermentable sugars production. Bioresour Technol 294:122–195
Karp SG, Schmitt CC, Moreira R, de Oliveira Penha R, de Mello AFM, Herrmann LW, Soccol CR (2022) Sugarcane biorefineries: status and perspectives in bioeconomy. BioEnergy Res 15(4):1842–1853
Galbe M, Wallberg O (2019) Pretreatment for biorefineries: a review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol Biofuels 12:1–26
Zetterholm J, Bryngemark E, Ahlström J, Söderholm P, Harvey S, Wetterlund E (2020) Economic evaluation of large-scale biorefinery deployment: a framework Integrating dynamic biomass market and techno-economic models. Sustainability 12:7126
Qiao J, Cui H, Wang M, Fu X, Wang X, Li X, Huang H (2022) Integrated biorefinery approaches for the industrialization of cellulosic ethanol fuel. Bioresour Technol 360:127516
Mashoko L, Mbohwa C, Thomas VM (2013) Life cycle inventory of electricity cogeneration from bagasse in the South African sugar industry. J Clean Prod 39:42–49
Akinbami OM, Oke SR, Bodunrin MO (2021) The state of renewable energy development in South Africa: an overview. Alex Eng J 60:5077–5093
Guna V, Ilangovan M, Akshay Koushik CV, Srinivasa CV, Nagananda GS, Venkatesh K, Reddy N (2019) Biofibers and biocomposites from sabai grass: a unique renewable resource. Carbohydr Polym 218:243–249
Patel A, Agrawal B, Rawal BR (2019) Pyrolysis of biomass for efficient extraction of biofuel. Energy Sources A: Recover Util Environ Eff 42:1649–1661
Rezania S, Oryani B, Cho J, Talaiekhozani A, Sabbagh F, Hashemi B, Rupani PF, Mohammadi AA (2020) Different pretreatment technologies of lignocellulosic biomass for bioethanol production: an overview. Energy 199:117457
Dadwal A, Sharma S, Satyanarayana T (2020) Progress in ameliorating beneficial characteristics of microbial cellulases by genetic engineering approaches for cellulose saccharification. Front Microbiol 11:1387
Yoo CG, Meng X, Pu Y, Ragauskas AJ (2020) The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: a comprehensive review. Bioresour Technol 301:122784
Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58
Meighana BN, Limaa DRS, Cardosoa WJ, Baêtaa BEL, Adarmea OFH, Santuccic BS, Pimentab MTB, de Aquinoa SF, Gurge LVA (2017) Two-stage fractionation of sugarcane bagasse by autohydrolysis and glycerol organosolv delignification in a lignocellulosic biorefinery concept. Ind Crops Prod 108:431–441
Zininga JT, Puri AK, Govender A, Singh S, Permaul K (2019) Concomitant production of chitosan and lipids from a newly isolated Mucor circinelloides ZSKP for biodiesel production. Bioresour Technol 272:545–551
Rodrigues Reis CE, Bento HB, Carvalho AK, Rajendran A, Hu B, De Castro HF (2019) Critical applications of Mucor circinelloides within a biorefinery context. Crit Rev Biotechnol 39:555–570
Carvalho AK, Bento HB, Reis CE, De Castro HF (2019) Sustainable enzymatic approaches in a fungal lipid biorefinery based in sugarcane bagasse hydrolysate as carbon source. Bioresour Technol 276:269–275
Zhang Y, Song Y (2021) Lipid accumulation by xylose metabolism engineered Mucor circinelloides strains on corn straw hydrolysate. Appl Biochem Biotechnol 193:856–868
Al Mousa AA, Hassane AM, Gomaa AE, Aljuriss JA, Dahmash ND, Abo-Dahab NF (2022) Response-surface statistical optimization of submerged fermentation for pectinase and cellulase production by Mucor circinelloides and M. hiemalis. Ferment 30:205
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2012) Determination of structural carbohydrates and lignin in biomass. Laboratory Analytical Procedure (LAP). Technical report NREL/TP-510–42618. National Renewable Energy Laboratory. pp 1–15. https://www.nrel.gov/docs/gen/fy13/42618.pdf. Accessed 12 July 2022
Qing Q, Zhou L, Huang M, Guo Q, He Y, Wang L, Zhang Y (2016) Improving enzymatic saccharification of bamboo shoot shell by alkalic salt pretreatment with H2O2. Bioresour Technol 201:230–236
Osipov DO, Dotsenko GS, Sinitsyna OA, Kondratieva EG, Zorov IN, Shashkov IA, Satrutdinov AD, Sinitsyn AP (2020) Comparative study of the convertibility of agricultural residues and other cellulose-containing materials in hydrolysis with Penicillium verruculosum cellulase complex. J Agron 10(11):1712
Alvarez MV, Ponce AG, Moreira MDR (2013) Antimicrobial efficiency of chitosan coating enriched with bioactive compounds to improve the safety of fresh cut broccoli. Food Sci Technol 50:78–87
Abugoch LE, Tapia C, Villamán MC, Yazdani-Pedram M, Díaz-Dosque M (2011) Characterization of quinoa protein–chitosan blend edible films. Food Hydrocoll 25:879–886
Mankar AR, Pandey A, Modak A, Pant KK (2021) Pretreatment of lignocellulosic biomass: a review on recent advances. Bioresour Technol 334:125–135
Chen Y, Stevens MA, Zhu Y, Holmes J, Xu H (2013) Understanding of alkaline pretreatment parameters for corn stover enzymatic saccharification. Biotechnol Biofuels 6:1–10
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Mesa L, González E, Cara C, González M, Castro E, Mussatto SI (2011) The effect of organosolv pretreatment variables on enzymatic hydrolysis of sugarcane bagasse. Chem Eng J 168:1157–1162
Novo LP, Gurgel LV, Marabezi K, Curvelo AA (2011) Delignification of sugarcane bagasse using glycerol-water mixtures to produce pulps for saccharification. Bioresour Technol 102:10040–10046
Luo X, Zeng B, Zhong Y, Chen J (2021) Production and detoxification of inhibitors during the destruction of lignocellulose spatial structure. BioResources 17:1939–1961
Yuan Y, Jiang B, Chen H, Wu W, Wu S, Jin Y, Xiao H (2021) Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol Biofuels 14:1–20
Demirba A (1998) Aqueous glycerol delignification of wood chips and ground wood. Bioresour Technol 63:179–185
Thoma C, Konnerth J, Sailer-Kronlachner W, Rosenau T, Potthast A, Solt P, van Herwijnen HWG (2020) Hydroxymethylfurfural and its derivatives: potential key reactants in adhesives. Chemsuschem 13:5408–5422
Troncoso-Ortega E, Castillo RDP, Reyes-Contreras P, Castano-Rivera P, Teixeira Mendonca R, Schiappacasse N, Parra C (2021) Effects on lignin redistribution in Eucalyptus globulus fibres pre-treated by steam explosion: a microscale study to cellulose accessibility. Biomolecules 11:507
Ning P, Yang G, Hu L, Sun J, Shi L, Zhou Y, Wang Z, Yang J (2021) Recent advances in the valorization of plant biomass. Biotechnol Biofuels 14:1–22
Rashid T, Kait CF, Murugesan T (2016) A “Fourier transformed infrared” compound study of lignin recovered from a formic acid process. Procedia Eng 148:1312–1319
Basiak E, Lenart A, Debeaufort F (2018) How glycerol and water contents affect the structural and functional properties of starch-based edible films. Polymers 10:1–18
Tarique J, Sapuan SM, Khalina A (2021) Effect of glycerol plasticizer loading on the physical, mechanical, thermal, and barrier properties of arrowroot (Maranta arundinacea) starch biopolymers. Sci Rep 11:1–17
Jeong J, Hussain F, Park S, Kang SJ, Kim J (2020) High thermal stability, high tensile strength, and good water barrier property of terpolyester containing biobased monomer for next-generation smart film application: Synthesis and characterization. Polymers 12:1–15
Zheng LY, Zhu JF (2003) Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr Polym 54:527–530
Goy RC, Britto DD, Assis OBG (2009) A review of the antimicrobial activity of chitosan. Polimeros 19:241–247
Moussa SH, Tayel AA, Al-Hassan AA, Farouk A (2013) Tetrazolium/Formazan test as an efficient method to determine fungal chitosan antimicrobial activity. J Mycol 2013:1–7
Duan C, Meng X, Meng J, Khan MIH, Dai L, Khan A, An X, Zhang J, Huq T, Ni Y (2019) Chitosan as a preservative for fruits and vegetables: a review on chemistry and antimicrobial properties. J Bioresour Bioprod 4:11–21
Huq T, Khan A, Brown D, Dhayagude N, He Z, Ni Y (2022) Sources, production and commercial applications of fungal chitosan: a review. J Bioresour Bioprod 7:85–98
Acknowledgements
The authors thank Ms. Anele Joyfull Dlamini for her assistance during some experiments.
Funding
Open access funding provided by Durban University of Technology. The authors received financial support from the National Research Foundation, Republic of South Africa (NRF/BRICS STI Grant No. 116019). A. K. P. also received postdoctoral fellowship from the Durban University of Technology during the course of this study.
Author information
Authors and Affiliations
Contributions
Johnson Tungamirai Zininga: laboratory work, writing — original draft; Adarsh Kumar Puri: methodology, co-supervision, data analysis, writing — review and editing; Nkosikho Dlangamandla: methodology, investigation; Zhengxiang Wang: resources, formal analysis, co-supervision; Suren Singh: co-supervision, project administration; Kugenthiren Permaul: conceptualization, project administration, methodology, writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval
No ethical approval was required as this study does not involve human and/or animal studies.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zininga, J.T., Puri, A.K., Dlangamandla, N. et al. Integrated biorefinery of Mucor circinelloides biomass and sugarcane bagasse for application of high-value biopolymers. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03935-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03935-1