Abstract
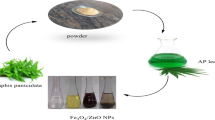
In this paper, two types of iron oxide nanomaterial (Fe3O4) and nanocomposite (T-Fe3O4) were created from the bio-waste mass of tangerine peel. These two materials were utilized for adsorption tests to remove cefixime (CFX) from an aqueous solution. Before the adsorption application, both adsorbents have been characterized by various characterizations such as XRD, FTIR, VSM, TEM, and FESEM. The mesoporous nano-crystalline structure of Fe3O4 and T-Fe3O4 nanocomposite with less than 100-nm diameter is confirmed. The adsorption of the obtained adsorbents was evaluated for CFX removal by adjusting several operation parameters to optimize the removal. The optimal conditions for CFX removal were found to be an initial concentration of 40 and 50 mg/L, a dosage of 0.25 mg/50 mL, a contact time of 120 min, and a pH of 5. These settings resulted in qe max values of 41.322 and 56.49 mg g−1 onto Fe3O4 and T-Fe3O4, respectively.




















Similar content being viewed by others
Data availability
All data used were mentioned in the manuscript and can be accessed.
References
Chaba JM, Nomngongo PN (2019) Effective adsorptive removal of amoxicillin from aqueous solutions and wastewater samples using zinc oxide coated carbon nanofiber composite. Emerg Contam 5:143–149. https://doi.org/10.1016/j.emcon.2019.04.001
Kareem SL, Jaber WS, Al-Maliki LA, Al-husseiny RA, Al-Mamoori SK, Alansari N (2021) Water quality assessment and phosphorus effect using water quality indices: Euphrates River- Iraq as a case study. Groundw Sustain Dev 14:100630. https://doi.org/10.1016/j.gsd.2021.100630
Fakhri A, Adami S (2014) Adsorption and thermodynamic study of Cephalosporins antibiotics from aqueous solution onto MgO nanoparticles. J Taiwan Inst Chem Eng 45(3):1001–1006. https://doi.org/10.1016/j.jtice.2013.09.028
Kareem S, Muheisen Al Mrayan AZ, Al-husseiny RA (2022) Human health risk assessment of heavy metals contaminated soil at Al-Nasiriyah City, Iraq. Egypt J Chem 65(9):1–3
Balarak D et al (2015) The use of low-cost adsorbent (canola residues) for the adsorption of methylene blue from aqueous solution: isotherm, kinetic and thermodynamic studies. Colloids Interface Sci Commun 7:16–19. https://doi.org/10.1016/j.colcom.2015.11.004
Samarghandi MR, Al-Musawi TJ, Mohseni-Bandpi A, Zarrabi M (2015) Adsorption of cephalexin from aqueous solution using natural zeolite and zeolite coated with manganese oxide nanoparticles. J Mol Liq 211:431–441. https://doi.org/10.1016/j.molliq.2015.06.067
Baaloudj O, Assadi I, Nasrallah N, El Jery A, Khezami L, Assadi AA (2021) Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: a review. J Water Process Eng 42:102089. https://doi.org/10.1016/j.jwpe.2021.102089
Esmaeili Bidhendi M, Poursorkh Z, Sereshti H, RashidiNodeh H, Rezania S, AfzalKamboh M (2020) Nano-size biomass derived from pomegranate peel for enhanced removal of cefixime antibiotic from aqueous media: kinetic, equilibrium and thermodynamic study. Int J Environ Res Public Health 17(12):4223. https://doi.org/10.3390/ijerph17124223
Baaloudj O, Nasrallah N, Bouallouche R, Kenfoud H, Khezami L, Assadi AA (2022) High efficient cefixime removal from water by the sillenite Bi12TiO20: photocatalytic mechanism and degradation pathway. J Clean Prod 330:129934. https://doi.org/10.1016/j.jclepro.2021.129934
Baaloudj O et al (2022) Techno-economic studies for a pilot-scale Bi12TiO20 based photocatalytic system for pharmaceutical wastewater treatment: from laboratory studies to commercial-scale applications. J Water Process Eng 48:102847. https://doi.org/10.1016/j.jwpe.2022.102847
Ciğeroğlu Z, Küçükyıldız G, Erim B, Alp E (2021) Easy preparation of magnetic nanoparticles-rGO-chitosan composite beads: optimization study on cefixime removal based on RSM and ANN by using genetic algorithm approach. J Mol Struct 1224:129182. https://doi.org/10.1016/j.molstruc.2020.129182
Al-husseiny RA, Ebrahim SE (2022) Effective removal of methylene blue from wastewater using magnetite / geopolymer composite : synthesis, characterization and column adsorption study. Inorg Chem Commun 139(February):109318. https://doi.org/10.1016/j.inoche.2022.109318
Badawi AK, AbdElkodous M, Ali GAM (2021) Recent advances in dye and metal ion removal using efficient adsorbents and novel nano-based materials: an overview. RSC Adv 11(58):36528–36553. https://doi.org/10.1039/D1RA06892J
Al-husseiny RA and Ebrahim SE (2021) Synthesis of geopolymer for the removal of hazardous waste : a review, https://doi.org/10.1088/1755-1315/779/1/012102
Lingamdinne LP, Vemula KR, Chang Y-Y, Yang J-K, Karri RR, Koduru JR (2020) Process optimization and modeling of lead removal using iron oxide nanocomposites generated from bio-waste mass. Chemosphere 243:125257. https://doi.org/10.1016/j.chemosphere.2019.125257
Al-husseiny RA, Ebrahim SE (2022) Synthesis of nano-magnetite and magnetite/synthetic geopolymer nano-porous composite for application as a novel adsorbent. Environ Nanotechnology Monit Manag. 18:100700. https://doi.org/10.1016/j.enmm.2022.100700
Rasoulifard MH, Khanmohammadi S, Heidari A (2016) Adsorption of cefixime from aqueous solutions using modified hardened paste of Portland cement by perlite; optimization by Taguchi method. Water Sci Technol a J Int Assoc Water Pollut Res. 74(5):1069–1078. https://doi.org/10.2166/wst.2016.230
Alkurdy FAR, Ebrahim SE (2020) Comparison between commercial and synthesized Fe3O4 nanoparticles for removal of heavy metal contaminants in wastewater. Assoc Arab Univ J Eng Sci. https://doi.org/10.33261/jaaru.2019.27.1.004
Zavareh S, Eghbalazar T (2017) Efficient and selective removal of cefixime form aqueous solution by a modified bionanocomposite. J Environ Chem Eng 5(4):3337–3347. https://doi.org/10.1016/j.jece.2017.06.042
Mohammed AA, Al-Musawi TJ, Kareem SL, Zarrabi M, Al-Ma’abreh AM, (2020) Simultaneous adsorption of tetracycline, amoxicillin, and ciprofloxacin by pistachio shell powder coated with zinc oxide nanoparticles. Arab J Chem 13(3):4629–4643
Tabatabaei FS et al (2020) Removal of cefixime from water using rice starch by response surface methodology. Avicenna J Med Biotechnol 12(4):230–235
Mohammed AA, Kareem SL (2019) Adsorption of tetracycline fom wastewater by using Pistachio shell coated with ZnO nanoparticles: equilibrium, kinetic and isotherm studies. Alexandria Eng J 58(3):917–928. https://doi.org/10.1016/j.aej.2019.08.006
Shaniba C, Akbar M, Ramseena K, Raveendran P, Narayanan BN, Ramakrishnan RM (2020) Sunlight-assisted oxidative degradation of cefixime antibiotic from aqueous medium using TiO2/nitrogen doped holey graphene nanocomposite as a high performance photocatalyst. J Environ Chem Eng 8(1):102204. https://doi.org/10.1016/j.jece.2018.02.012
Mohammed AA, Kareem SL (2021) Enhancement of ciprofloxacin antibiotic removal from aqueous solution using ZnO nanoparticles coated on pistachio shell. Desalin WATER Treat 213:229–239
Mohammed AA, Kareem SL, Peters RJ, Mahdi K (2022) Removal of amoxicillin from aqueous solution in batch and circulated fluidized bed system using zinc oxide nanoparticles: hydrodynamic and mass transfer studies. Environ Nanotechnology Monit Manag 17:100648. https://doi.org/10.1016/j.enmm.2022.100648
Azimi Bizaki S, Bahramifar N, Zandipak R, and Bahmeei F (2020) Preparation of magnetic graphene nanocomposite for the removal of cefixime from aqueous solution, Int J Environ Anal Chem, 1–17, https://doi.org/10.1080/03067319.2020.1838496
Mohseni-Bandpei A, Eslami A, Kazemian H, Zarrabi M, Venkataraman S, Sadani M (2023) Enhanced adsorption and recyclability of surface modified hydrophobic silica aerogel with triethoxysilane: removal of cefixime by batch and column mode techniques. Environ Sci Pollut Res 30(1):1562–1578. https://doi.org/10.1007/s11356-022-22277-5
Naghipour D, Amouei A, Taher K, and Taghavi K (2020) Removal of cefixime from aqueous solutions by the biosorbent prepared from pine cones : kinetic and isotherm studies, 201: 219–227, https://doi.org/10.5004/dwt.2020.26133
Hasanzadeh V, Rahmanian O, Heidari M (2020) Cefixime adsorption onto activated carbon prepared by dry thermochemical activation of date fruit residues. Microchem J 152:104261. https://doi.org/10.1016/j.microc.2019.104261
Pham TD et al (2020) Adsorption characteristics of beta-lactam cefixime onto nanosilica fabricated from rice HUSK with surface modification by polyelectrolyte. J Mol Liq 298(xxxx):111981. https://doi.org/10.1016/j.molliq.2019.111981
Farhan AM, Salem NM, Ahmad AL, Awwad AM (2012) Kinetic, equilibrium and thermodynamic studies of the biosorption of heavy metals by Ceratonia siliqua bark. Am J Chem 2(6):335–342
Abdi O, Kazemi M (2015) A review study of biosorption of heavy metals and comparison between different biosorbents. J Mater Env Sci 6(5):1386–1399
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–59
Sayğılı GA, Sayğılı H, Koyuncu F, Güzel F (2015) Development and physicochemical characterization of a new magnetic nanocomposite as an economic antibiotic remover. Process Saf Environ Prot 94:441–451
Gao B, Li P, Yang R, Li A, Yang H (2019) Investigation of multiple adsorption mechanisms for efficient removal of ofloxacin from water using lignin-based adsorbents. Sci Rep 9(1):637. https://doi.org/10.1038/s41598-018-37206-1
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by Rasha A. Al-husseiny and Shahlaa E. Ebrahim. Result analysis and models were performed by Sabreen L. Kareem. The first draft of manuscript was written by Rasha A. Al-husseiny and Sabreen L. Kareem. Finally, technical aspects were done by Ahmed Naji. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This declaration is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Al-husseiny, R.A., Kareem, S.L., Naje, A.S. et al. Effect of green synthesis of Fe3O4 nanomaterial on the removal of cefixime from aqueous solution. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-03921-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-03921-7