Abstract
Plastic waste in Egypt is causing a problem. An innovative solution to reduce this pollution is inevitable. Microalgae were considered a new resource for developing biobased bioplastics. This study aims to prepare a simple biodegradable film able to use in packaging and from polyurethane (PU) nanocomposite with Poly 3-hydroxybutyrate (PHB) in the presence of various concentrations of CuO-NPs. The Poly 3-hydroxybutyrate compounds were extracted from the dominant microalgal species in High rate algal pond Microcystis sp. Algal extracts have antimicrobial activities against Escherichia coli, Salmonella Typhimurium, Pseudomonas aeruginosa, Staphylococcus aureus, Listeria monocytogenes, Enterococcus faecalis, and Candida albicans. Films prepared from PHB, and PU showed no cytotoxic impact on human tumor cell lines in terms of cell viability. The mechanical properties of the films were studied, and it was found that the final contact angle values were improved from 77° to 87° as CuO-NPs loading raised from 2 to 4%, respectively. XRD displays no difference in the intensity of CuO-NPs peaks by increasing CuO-NPs loading in the PHB/PU matrix. FTIR spectra of all blends were recorded in the range of 400 to 4000 cm−1. Tensile properties were improved with the addition of 40 wt.% PHB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
More plastic is being used, which results in daily increases in environmental contamination. Research on bio-based plastic is now receiving attention in an effort to create a sustainable, greener world with a smaller environmental impact. As long as additional waste management features, like composting, are included, bio-based and biodegradable bioplastics can offer similar properties to traditional plastics while also generating additional returns due to their reduced carbon footprint. The use of non-degradable polymers for packaging in the Egyptian environment is one of the biggest problems affecting the environment [1]. The idea of replacing the non-degradable polymers with other biodegradable and environmentally friendly polymers is a new concept to the Egyptian market. Despite the importance of using biodegradable polymers in packaging, this technology is expensive, from this point of view; the search for alternatives or solutions to reduce this environmental problem is a priority for the Egyptian research community [2, 3]. The term “biodegradable polymers” or “biopolymers” refers to polymeric substances that can break down by the enzymatic action of microorganisms like bacteria, fungi, and algae in a bioactive environment. The most important components of biobased products like bioplastics, biopolymers, and biobased polyurethane are lipids, proteins, and carbohydrates, which microalgae and some cyanobacteria species are capable of manufacturing in large quantities [4, 5]. In recent years, microalgal biomass has been identified as a possible source of materials for enhancing a number of disciplines, including the manufacturing of bioplastics [6, 7]. This biomass can be used either directly as biomass or as a feedstock for secondary processes. As an internal carbon source from sugar and/or lipids, bacterial and algal cells make polyhydroxyalkanoates (PHAs), and polyesters from hydroxyalkanoates [8]. Given their similar physical qualities, PHAs are seen as potential alternatives to petrochemical-based plastics like polypropylene in plastic bags and containers [8, 9]. Poly 3-hydroxybutyrate (PHB), is one of the most studied and commercially available short-chain-length PHAs produced by microalgae [10,11,12]. Polymer mixing is a straightforward method that produces better polymeric materials. PHA blends have been the subject of numerous investigations to enhance their qualities and lower production costs [13].
Given their structure–property correlations, polyurethanes (PUs) are a family of adaptable materials with a lot of promise for usage in various applications. The ability to customize PUs for usage in many applications is receiving major research attention due to their unique mechanical, physical, biological, and chemical properties.
The characteristics and performance of PU-based materials can be improved by altering the manufacturing procedure, the raw ingredients used to make them, or by using cutting-edge characterization methods. It is obvious that altering the raw ingredients and production process using the right techniques might result in PUs that are appropriate for a variety of specialized applications [13].
The features of PUs include outstanding gloss, color retention, strong impact resistance, high tensile strength, high abrasion resistance, and excellent elasticity and elongation [14]. They can be used in a number of sectors, such as leather [15], foams [16,17,18], furniture, fibers [19,20,21], elastomers [22], adhesives [23], paints [24, 25], coatings [26], sensors [27,28,29,30], electrical components [28], biomedical [29, 30], and others [31, 32] thanks to the aforementioned qualities. Despite having many uses, the usage of freestanding PU frequently makes it impossible to achieve adequate thermal and mechanical capabilities. These factors make character augmentation in PUs by blending and composite type inevitable.
What occurs when conducting polymers are introduced to insulating matrices like PU is already well understood. In many different applications, such as catalysts, ceramics, thermoelectric sensors, glass, superconducting materials, and antibacterial compounds, copper oxide nanoparticles (CuO-NPs) are widely used [29]. CuO-NPs are less expensive to produce than gold and silver nanoparticles. CuO-NPs' unique crystal structure contributes to their potent antibacterial properties as well. Size, liquid aggregation, surface charge, and other physical and chemical characteristics of NPs, as well as their final interactions with target cells, are all impacted by these characteristics. CuO-NPs have been demonstrated to have a substantial antibacterial effect against E. coli and Staphylococcus, depending on the concentration and particle size [30].
Copper nanocomposites have been shown to be effective at suppressing E. coli in numerous studies [31], and their effectiveness rises with increasing concentration and contact time. Compared to film treated with NaBH4, film containing heat-treated CuO-NPs shows superior antibacterial capabilities against the growth of E. coli [32]. Gram-negative and Gram-positive bacteria are not resistant to CuO-NPs’ antibacterial activities, albeit their antibacterial potency was reduced when they were included in bacterial cellulose nanofiber [33].
The purpose of this study is to outline the extended applications of polyurethane (PU)-based nanocomposites incorporated with poly 3-hydroxybutyrate (PHB) extracted from microalgae that is grown in a high-rate algal pond used in wastewater treatment, in presence of different ratios of CuO-NPs to fabricate PHB/PU/CuO bionanocomposite with the aim of producing biodegradable films that can be used in packaging.
2 Materials and methods
2.1 Materials
Pellets of polyurethane (PU) with a specific gravity of 1.20 g/cm3 were purchased from Bayer in Germany.
The copper oxide nanoparticle (CuO-NPs) came from the American company Sigma Aldrich.
(Poly (3-hydroxybutyrate) was obtained from Sigma Aldrich in the United States and dissolved in chloroform (80 °C.) within 45 to 60 min while in reflux.
2.2 Microalgae harvesting and processing
The high-rate algal pond (HRAP), which was built at the Zenin wastewater treatment facility in Giza, 100 ml chloroform Egypt, was used to treat wastewater and harvest microalgae. Al SO4 was used to coagulate the microalgae before they were harvested, dried in a sun drier at a temperature of 40 to 45 °C, and kept at a temperature of 20 °C.
2.3 Microalgal community structure
The algal sample was preserved using a Lugol’s iodine solution. The OLYMPUS CX41 microscope was used to analyze sub-samples after they were distributed into glass Sedgwick-Rafter cells. Semi-quantitative analysis was used to determine the samples' species composition and dominance. Algal identification has been carried out in accordance with the primary sources utilized to identify phytoplankton [34].
2.4 Preparation of microalgal PHB extract
Algal biomass (collected from HRAP) was suspended in sterile water and homogenized then allowed for mixing with a vortex. 2 N HCL was added then heated for 2 h in a water bath. Then, centrifuged at 6000 rpm for 20 min, and chloroform is added and left overnight at 28 °C on a shaker at 150 rpm. Then, it was centrifuged at 2000 rpm for 20 min and dried at 40 °C [35].
2.5 Preparation of PHB/PU/CuO bionanocomposites
The PHB/PU/CuO bionanocomposites were prepared by dissolving 3 g of PU in 100 ml chloroform and 3 g of the prepared PHB was dissolved also in 100 ml chloroform and then added to PU solution by (40:60) to form PHB/PU blend. Then CuO-NPs were added to the PHB/PU blend by the following ratios (1, 2, and 4%) and the PHB/PU/CuO bionanocomposites films by casting method [36]
2.6 Antimicrobial activity
The antimicrobial activity of the algal extract (0.1, 0.5, and 1 mg/L) against different pathogenic microorganisms was carried out using disc diffusion method according to [37]. E. coli (ATCC 25,922), Salmonella enterica serovar Typhimurium (ATCC 14,028), and Pseudomonas aeruginosa (ATCC 10,145) as Gram-negative bacteria, Moreover, Staphylococcus aureus (ATCC 43,300), Enterococcus faecalis (ATCC 51,299), and Listeria monocytogenes (ATCC 25,152) as Gram-positive bacteria and Candida albicans (ATCC 10,231) as a yeast were used in this study. Sterile disc (6 mm in diameter) was immersed in the algal suspension and left for 2 min for saturated with the suspension. The saturated disc with algal extract was transferred to the Müller-Hinton agar plate that was previously inoculated by the tested pathogenic microorganisms on the agar surface using the spread method. The plates were incubated at 37 °C for 24–48 h. The inhibition zones were measured in mm using a measuring ruler. Six mm inhibition zone diameters showed no any antimicrobial effect.
2.7 Cytotoxicity effect on human cell lines
Cytotoxic impact of prepared films on human tumor cell lines in terms of cell viability was estimated by the mitochondrial-dependent reduction of yellow 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to purple formazan. All the following experiments were also performed in a sterile area using a Laminar flow biosafety cabinet class II (manufactured by Labconco). In brief, human hepatocellular carcinoma cell line (HePG2) cells were suspended in DMEM medium, 1% antibiotic–antimycotic mixture (10,000 U/ml potassium penicillin, 10,000 µg/ml streptomycin sulfate and 25 µg/ml amphotericin B) and 1% L-glutamine and 5% fetal bovine serum at 37 °C under 5% CO2 using CO2 incubator (Sartorius Stedim, Biotech, Göttingen, Germany). Viable cells were batch cultured for 10 days, then seeded at a concentration of 10 × 103 cells/well in fresh complete growth medium in 96-well plastic plates at 37 °C for 24 h under 5% CO2 either alone (negative control) or with different concentrations of drugs to give a final concentration of (500, 250, 125, 62.5, 31.25 μg/ml). After 48 h of incubation, the medium was aspirated, 20 μl MTT salt (2.5 μg/ml) were added to each well and incubated for further 4 h at 37 °C under 5% CO2. To stop the reaction and dissolve the formed crystals, 200μL of 10% sodium dodecyl sulfate (SDS) in 0.01 M HCL was added to each well and incubated overnight at 37 °C. A positive control which composed of 100 µg/ml was used as a known cytotoxic natural agent that gives 100% lethality under the same condition. The absorbance was then measured using a microplate multi-well reader (Bio-Rad Laboratories Inc., model 3350, Hercules, California, USA) at 595 nm and a reference wavelength of 620 nm [37, 38].
2.8 Characterizations of bionanocomposites
2.8.1 X-ray diffraction (XRD)
The XRD patterns of the bionanocomposites were carried out on a Diano X-ray diffractometer using CoKα radiation source energized at 45 kV and a Philips X-ray diffractometer (PW1930 generator, PW 1820 goniometer) with radiation of Cu Kα source (45 kV, 40 mA, with λ = 0.15418 nm) with 2θ range of 5 to 80° over the size of a step of 0.02 and time of the step of 1 s. The basal spacing (d) was calculated from Bragg’s equation,

where n is an integer, ƛ is the wavelength of incident waves, d is the distance between the planes in the atomic lattice, and ɵ is the angle between the incident ray and the scattering planes [36].
2.8.2 Scanning electron microscopy (SEM)
The morphology of the synthesized films was viewed by SEM, (JSM 6360LV, JEOL/Noran). The images were obtained using an accelerating voltage of 10–15 kV. Before examination, the samples were sputter coated with a fine layer of gold in a low deposition rate, refrigerated, and placed at the maximum distance from the target to prevent damage [36].
2.8.3 Contact angle measurement
The surface tension of the bio-polymers was evaluated via a video-based optical contact angle measuring system (OCA 20 Dataphysics) which was used to determine the contact angle according to DIN ISO 55660–1/2. The measurements were taken at room temperature and at constant humidity and the drop volume was held constant.
2.8.4 Fourier transforms infrared (FT-IR) spectroscopy
FT-IR spectra of PHB/PU blend and PHB/PU/CuO bionanocomposites which containing different ratios of CuO-NPs (1%, 2%, and 4%) were recorded in the range 500–4000 cm−1 on (Shimadzu 8400S) FT-IR [36].
2.9 Mechanical properties of the prepared bionanocomposites films
The mechanical features of the fabricated PHB/PU/CuO bionanocomposites were measured in accordance with the ASTM standard of D638-91 with an LK10k as a universal testing machine fixed with a load cell of 5 K N, and it operated at a rate of 10 mm/min on the samples.
2.9.1 Barrier properties
By making use of a GBPI W303 (B) Water Vapor Permeability Analyzer, the water vapor transmission rate was measured using the cup method. The water vapor transmission rate (WVTR) was calculated in terms of the amount of water vapor that has moved in a unit time across the unit area under specific humidity (4–10%) and temperature (38 °C) conditions as per the standards of JIS Z0208, 53,122–1, TAPPI T464, ASTM D1653, ISO 2528, and ASTM E96. Moreover, the rate of transmission of gas GTR (O2) is obtained by means of an N530 B Gas Permeability Analyzer (China), as per the standards followed by ASTM D1434-82 (2003).
3 Results and discussion
3.1 Microalgal identification
The species that made up the microalgal assemblage were mostly from the phylum Cyanophyta, while there were also few from the phyla Bacillariophyta and Chlorophyta. All species detected in HRAP during the course of this investigation were recognized to be important members of the microalgal communities with represented dominant genera Microcystis sp. which is belonging to Cyanophyta [35]. Despite that, there was the rare presence of some types of green algae represented in Scenedismus sp., Pediastrum sp., and Oocystis sp.
3.2 Identification of PHB using NMR
1HNMR (CDCl3, 400 MHz) showed a characteristic peak at δ 5.1936 ppm confirming the presence of protons of hydroxylated carbons, in addition to several multiplets representing aliphatic CH groups in the range δ 2–3.7 ppm. The carbonyl function of the PHA was confirmed by 13CNMR at δ 179 and 182 ppm as shown in Fig. 1. In addition, PHB was detected using a spectrophotometer at 235 nm.
3.3 Antimicrobial activity
The disc diffusion assay is commonly used for the determination of the antibacterial and antifungal activities of micro and macro algae and marine algal extracts [38]. In this study, the concentrations (0.1, 0.5, 1.0 mg/L) of the algal extract have potent antimicrobial activities against three Gram-negative bacteria (E. coli, Salmonella Typhimurium, and Psudomonas aeruginosa), three Gram-positive bacteria (Staphylococcus aureus, Listeria monocytogenes, and Enterococcus faecalis) and Candida albicans as shown in Fig. 2. The most susceptible microorganisms to algal extract were Enterococcus faecalis followed by Salmonella Typhimurium and then E. coli. While the relatively resistant stain to algal extract was Candida albicans, whereas 0.1 mg/L of algal extract has no antimicrobial effect against Candida albicans. Many studies stated that microalgal extracts such as poly (3-hydroxybutyrate) (PHB) have excellent antimicrobial effects against Gram-negative bacteria, Gram-positive bacteria, and fungi [39, 40].
3.4 Cytotoxicity assessment
The cytotoxicity assessment of human tumor cell lines was verified by determining the percentage cell viability and IC50 values using the MTT assay as displayed in Fig. 3A–D. The investigated samples were tested at different concentrations ranging from 500 to 31.25 μg/mL under incubation at 37 °C/5% CO2 for 48 h. As illustrated in (Fig. 3A), the cell viability was located in the range of 80–100% even at all studied concentrations. This confirms that the prepared films either with or without algal extract did not exhibit cytotoxicity to the cells. Moreover, Serini et al. [41] reported that the material can be exhibited a significant toxicity when the cell viability value was less than 50% compared to the control cells. In all most cases for our data which the cytotoxicity was measured, the cell viability was above 90%, indicating that these biomaterials are extremely safe and can be a promising candidate for safe packaging. Consistently, adding algal extract in the matrix could potentially decrease the IC50 value (Fig. 6c) [31].
3.5 SEM analysis
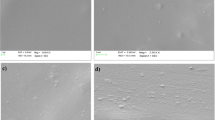
Figure 4 shows surface microstructure micrographs of bioplastic films. Surface features of control showed uniform surface. While features of bioplastic films which contain algal extract were ununiform and contained voids. This occurred because the forming procedure did not fully gelatinize the algal extract. This result is consistent with Hernandez et al., reporting [42], the SEM images exhibited contain insoluble remains (also known as ghosts) form. On the broken surface, several cavities were also discernible (black dots are pictured), which may have contributed to the material's low impact and tensile strength.
3.6 Final contact angle
The final contact angle of the pure polyurethane (PU), polyhydroxybutyrate (PHB) film as well as PHB/PU/CuO bionanocomposites containing different ratios of CuO-NPs (2, and 4%) is displayed in (Fig. 5). A contact angle experiment was conducted to find out whether the prepared bioplastic films were hydrophobic in nature or hydrophilic in nature [41, 42]. The achieved data in Table 1, revealed that the final contact angle for PU is around 68° and the contact angle for PHB/PU film is 72° that revealed the PHB/PU film has poor resistance to water because of the occurrence of hydroxyl groups in the prepared PHB/PU film for that reason the surface of the fabricated PHB/PU film is partially hydrophobic. Furthermore, the final contact angle values were improved from 77° to 87° as CuO-NPs loading raised from 2 to 4%, respectively. Depending on how moist the solid substance is, the contact angle might range from 0 to 180 degrees. The 0° implies that the substance is extremely hydrophilic, whereas the 180° indicates that the material is hydrophobic [43].
The obtained results display that through increasing the concentration of CuO-NPs into the bionanocomposites matrix has a propensity to avoid the distribution of the water drop above the surface of the prepared PHB/PU/CuO bionanocomposites film following in an increasing of the hydrophobicity properties of PHB/PU/CuO bionanocomposites film surfaces as displaying in (Fig. 5).
3.7 X-ray diffraction (XRD)
The X-ray diffraction patterns of the prepared PHB/PU blend film and the prepared PHB/PU/CuO bionanocomposites films loading with different CuO-NPs are revealed in (Fig. 6). The obtained results in Fig. 6 displayed that the XRD pattern for 2θ angles between 4 and 80° for all prepared samples, Fig. 6a showed the diffraction pattern of the fabricated PHB/PU blend films which appear in amorphous form. The X-ray pattern showed a justly well-matched monoclinic structure for CuO-NPs. The characteristic peaks positioned at 2 theta degree = 31.65°, 34.95°, 42.50°, and 48.89° are apportioned to (110), (002), (200), and (202) plane orientation of CuO nanoparticles (JCPDS 80–1268). These results were in accordance with results reported by other researcher [44, 45] Moreover, the XRD displays that there is no difference in the intensity of CuO-NPs peaks by increasing of CuO-NPs loading in the PHB/PU matrix as revealed in Fig. 6.
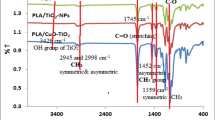
3.8 FT-IR spectroscopy of the prepared PHB/PU/CuO bionanocomposites
Fourier transforms infrared spectra of PHB/PU blend and PHB/PU/CuO bionanocomposites with and without the addition of CuO-NPs with different ratios (0.5, 1.0, 2.0, and 4.0%), respectively, were recorded in the range 400 to 4000 cm1 to begin possible intermolecular interactions among various constituents in the structure of the created bionanocomposites. According to Fig. 7, the spectrum of the PHB/PU/CuO bionanocomposites films revealed no differences in the structure of the CuO nanoparticles, polyurethane (PU) film, polyhydroxybutyrate (PHB), or the created PHB/PU blend. Additionally, the PHB/PU mixture exhibits FT-IR absorption peaks at 3283 and 2937 cm−1, which are associated with the (OH) stretching and (CH2) asymmetric stretching groups, with the bands for the PU appearing at 3315 cm−1 (N–H group).Additionally, IR spectroscopy showed substantial absorptions indicative of PHB at 1754 cm-1 and 1247 cm-1, respectively, confirming the existence of C = O and C-O stretching groups.
Additionally, the broad peak seen at 3340 cm-1 is connected to the stretching of OH from intra- and intermolecular hydrogen bonding. A method that may analyze samples made up of different materials is called Fourier transform infrared spectroscopy (FTIR) [46, 47].
3.9 Mechanical properties
In order to reduce the projected packaging material risk during handling and transit and thus increase food preservation, the mechanical properties of the proposed bionanocomposites materials are essential. The mechanical characteristics of PHB/PU/CuO bionanocomposites were therefore examined to demonstrate the impact of CuO-NPs addition at various amounts (1, 2, and 4%). The results shown in Table 2 revealed that Pure PU film had a tensile strength of 5.69 MPa and high elongation at a break of 187.29%. With the addition of 40 weight percent PHB, both tensile characteristics increased to 6.24 MPa and 285.86%, respectively.
Additionally, by adding CuO-NPs to the PHB/PU blend in various concentrations, the mechanical properties of the created PHB/PU/CuO bionanocomposites were improved. For example, the tensile strength increased from 6.24 MPa for PHB/PU blend to 7.04 MPa with the addition of (1.0%) CuO-NPs, and it also increased to 11.30 and 13.27 MPa by increasing the CuO-NPs concentrations from 2 to 4%.
Additionally, the addition of CuO-NPs to the PHB/PU blend improved the elongation of the synthesized PHB/PU/CuO bionanocomposites from 285.86 to 308.74%. The elongation was also enhanced by increasing the loading of CuO-NPs from 2 to 4% in the PHB/PU blend from 377.95 to 412.69%. The enhanced mechanical characteristics of the produced PHB/PU/CuO bionanocomposites were mostly attributable to the reinforcement of CuO nanoparticles with greater compatibility and dispersion inside the PHB/PU matrix.
This result indicates that the incorporation of CuO-NPs increases the interfacial adhesion, which may be a crucial factor in creating compatibilization at a molecular level. Tensile strength is dependent on the interface between the polymer matrix and filler. CuO-NPs compatibility enhances stress transfer inside PHB/PU/CuO bionanocomposites, increasing tensile strength. The creation of inorganic clusters or agglomerates may be enhanced by further addition of high nanofiller content, which lowers the tensile strength. This increase may be caused by the CuO-NPs surface's interaction with the polymer chains, which results in examples which are rigid and break easily with little elongation at break. Higher elongation is currently seen, which is attributed to both good interfacial adhesion around the CuO-NPs and good dispersion of the major nanoscale CuO-NPs particles that limit polymer chain mobility under loading. The findings demonstrated that plastic films’ qualities are significantly superior to those of commercial plastic, where the elongation of commercial plastic bags is 222.5% [48].
3.9.1 Water vapor transmission rate (WVTR) evaluation
Barrier qualities are thought to be crucial when packaging food. They may be affected by certain traits including compatibility, filler dispersion, and material crystallinity. The WVTR values are shown in Table 3. Pure PU and its blend with PHB both had substantially better WVTR barrier qualities, with WVTR values of 93.79 and 90.70 g/m2 day, respectively. Despite a marked drop in WVTR values, PHB/PU/CuO-NPs bionanocomposites based on metal-chelation nevertheless reached 68.65 g/m2 day, notably in the case of PHB/PU/CuO-NPs containing 4% CuO-NPs.
This drop was attributed to the blend's high crystallinity, which increased tortuosity in the matrix and decreased water vapor transfer, as well as good miscibility (i.e., a smooth surface) and nanodispersion. Additionally, this finding demonstrates how very potential PHB/PU/CuO-NPs bionanocomposites are for use in food packaging. This is the situation due to the compact nature of nanofiller aggregates. This result was consistent with the mechanical discoveries covered in the previous section.
4 Conclusions
-
The algal extract used in bionanocomposites films has anti-bacterial properties and it has improved the mechanical properties of the films, which enables it to be used in many applications.
-
The bioplastic films, which were made of the bionanocomposites in addition to algal extract, can be used as commercial plastic packaging.
-
The results of this study have met the standard.
-
The developed biocomposite materials are good at preventing the expected risks of packaging materials during use and transportation, which leads to more food preservation.
-
Using biodegradable packaging in daily or commercial use can preserve habitat in the environment and protect biological systems.
Data availability
The date used to support the finding of this study is included in the manuscript.
References
Mohamed NA (2015) Promoting of Environment Friendly Packaging Utilizing in the Egyptian Market “Study on Grocery Bags by Carrefour Egypt.” Civil Environ Res 7(4):54–61
Elhussieny A, Faisal M, D’Angelo G, Aboulkhair NT, Everitt NM, Fahim IS (2020) Valorisation of shrimp and rice straw waste into food packaging applications. Ain Shams Eng J 11(4):1219–1226
Khan FR, Shashoua Y, Crawford A, Drury A, Sheppard K, Stewart K, Sculthorp T (2020) ‘The plastic nile’: First evidence of microplastic contamination in fish from the nile river (Cairo, Egypt). Toxics 8(2):22
Martins A, Caetano NS, Mata TM (2010) Microalgae for Biodisel Production and Other Applications: A Review. Renewable Sustain Energy Rev 14:217–232
Mehta P, Singh D, Saxena R, Rani R, Gupta RP, Puri SK, Mathur AS (2018) High-Value Coproducts from Algae—An Innovational Way to Deal with Advance Algal Industry. In Waste to Wealth; Singhania, R., Agarwal, R., Kumar, R., Sukumaran, R., Eds.; Springer: Singapore, pp. 343–363. ISBN 9789811074318
Rahman A, Miller CD (2017) Microalgae as a Source of Bioplastics. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands 121–138. ISBN 9780444637840
Madadi R, Maljaee H, Serafim LS, Ventura SP (2021) Microalgae as contributors to produce biopolymers. Mar Drugs 19(8):466
Kaparapu J (2018) Polyhydroxyalkanoate (PHA) Production by Genetically Engineered Microalgae: A Review. J New Biol Rep 7:68–73
Lambert S, Wagner M (2017) Environmental Performance of Bio-Based and Biodegradable Plastics: The Road Ahead. Chem Soc Rev 46:6855–6871
Balaji S, Gopi K, Muthuvelan B (2013) Review Article: A Review on Production of Poly β hydroxybutyrates from Cyanobacteria for the Production of Bioplastics. ALGAL 2:278–285
Kovalcik AA, Meixner K, Zeilinger W, Fritz I, Fuchs W, Stelzer F, Drosg B, Zeilinger W, Fritz I, Fuchs W et al (2017) Characterization of Polyhydroxyalkanoates Produced by Synechocystis salina from Digestate Supernatant. Int J Biol Macromol 102:497–504
Roja K, Sudhakar DR, Anto S, Mathimani T (2019) Extraction and Characterization of Polyhydroxyalkanoates from Marine Green Alga and Cyanobacteria. Biocatal Agric Biotechnol 22:101358
Li Z, Yang J, Loh XJ (2016) Polyhydroxyalkanoates: Opening Doors for a Sustainable Future. NPG Asia Mater 8:e265-20
Qin M, Chen C, Song B, Shen M, Cao W, Yang H, Zeng G, Gong J (2021) A review of biodegradable plastics to biodegradable microplastics: Another ecological threat to soil environments? J Clean Prod 312:127816
Moshood TD, Nawanir G, Mahmud F, Mohamad F, Ahmad MH, AbdulGhani A (2022) Biodegradable plastic applications towards sustainability: A recent innovations in the green product. Clean Eng Technol 6:100404
Moshood TD, Nawanir G, Mahmud F, Mohamad F, Ahmad MH, AbdulGhani A (2022) Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr Res Green Sustain Chem 5:100273
El-malek FA, Khairy H, Farag A, Omar S (2020) The sustainability of microbial bioplastics, production and applications. Int J BiolMacromol 157:319–328. https://doi.org/10.1016/j.ijbiomac.2020.04.076
Akindoyo JO, Beg M, Ghazali S, Islam MR, Jeyaratnam N, Yuvaraj AR (2016) Polyurethane types, synthesis and applications–a review. RSC Adv 6(115):114453–114482
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of frst and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev. https://doi.org/10.1016/j.rser.2009.10.003
Khatoon H, Ahmad S (2017) A review on conducting polymer reinforced polyurethane composites. J Ind Eng Chem 53:1–22
Zhu X, Li Q, Wang L, Wang W, Liu S, Wang C, Xu Z, Liu L, Qian X (2021) Current advances of polyurethane/graphene composites and its prospects in synthetic leather: A review. Eur. Polym. J. 161:110837
Lubczk R, Broda D, Kus-Li´skiewicz M, Szczech D, Bobko E, Debska B, Szpiłyk M, Lubczak J (2021) Flame retardant polyurethane foams with starch unit. Polym Test 104:107395
Moon JH, Kwak SB, Lee JY, Kim DY, Ha JU, Oh JS (2019) Synthesis of polyurethane foam from ultrasonically decrosslinked automotive seat cushions. Waste Manag 85:557–562
Kim JH, Choe H (2019) Reactivity of isophorone diisocyanate in fabrications of polyurethane foams for improved acoustic and mechanical properties. J Ind Eng Chem 69:153–160
Sun W, Jia L, Wang Z, Jia Z (2018) Optical fiber sensor encapsulated by polyurethane. Optik 165:124–131
Qian HF, Feng G, Bai G, Liu YC, Hu LL (2017) A contrastive study of adsorption behaviors on polyurethane fiber with diester/diurethane tethered and non-tethered azo disperse dys. Dyes Pigm 145:301–306
Choi JH, Moon DS, Ryu SH, Lee BJ, Ying YB, Lee KJ (2018) N-chloro hydantoin functionalized polyurethane fibers toward protective cloth against chemical warfare agents. Polymer 138:146–155
Wang F, Chen S, Wu Q, Zhang R, Sun P (2019) Strain-induced structural and dynamic changes in segmented polyurethane elastomers. Polymer 163:154–161
Tenorio-Alfonso A, Sánchez MC, Franco JM (2021) Impact of moisture curing conditions on the chemical structure and rheological and ultimate adhesion properties of polyurethane adhesives based on castor oil and cellulose acetate. Prog Org Coat 161:106547
Carreño F, Gude MR, Calvo S, de la Fuente OR, Carmona N (2020) Design and development of icephobic coatings based on sol-gel/modified polyurethane paints. Mater Today Commun 25:101616
Das A, Mahanwar P (2020) A brief discussion on advances in polyurethane applications. Adv Ind Polym Res 3:93–101
Raychura AJ, Jauhari S, Prajapati VS, Dholakiya BZ (2018) Synthesis and performance evaluation of vegetable oil based wood finish polyurethane coating. Bioresour Technol Rep 3:88–94
Li R, Ton Loontjens JAT, Shan Z (2019) The varying mass ratios of soft and hard segments in waterborne polyurethane films: Performances of thermal conductivity and adhesive properties. Eur Polym J112:423–432
Dang X, Li Y, Yang M (2019) Biodegradable waterborne polyurethane grafted with gelatin hydrolysate via solbent-free copolymerization for potential porous scaffold material. J Mech Behav Biomed 92:79–89
Ijaz F, Shahid S, Khan SA, Ahmad W, Zaman S (2017) Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activities. Trop J Pharm Res 16:743–753. https://doi.org/10.4314/tjpr.v16i4.2
Shahzadi S, Zafar N, Sharif R (2018) Antibacterial activity of metallic nanoparticles. Bacterial Pathogenesis and Antibacterial Control, 51. https://doi.org/10.5772/intechopen.72526
Harikumar PS, Aravind A (2016) Antibacterial activity of copper nanoparticles and copper nanocomposites against Escherichia coli bacteria. Int J Sci 5(2):83–90. https://doi.org/10.1016/j.tox.2013.07.012
Gautam G, Mishra P (2017) Development and characterization of copper nanocomposite containing bilayer film for coconut oil packaging. J Food Process Preserv 41:e13243. https://doi.org/10.1111/jfpp.13243
Almasi H, Jafarzadeh P, Mehryar L (2018) Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties. Carbohydr Polymer 186:273–281. https://doi.org/10.1016/j.carbpol.2018.01.067
Doma H, Moghazy R, Mahmoud R (2021) Environmental factors controlling algal species succession in High Rate Algal Pond. Egypt J Chem 64(2):729–738
Abdo SM, Ali GH (2019) Analysis of polyhydroxybutrate and bioplastic production from microalgae. Bullet Natl Res Centre 43(1):1–4
Abdelhak A, Ghanem AF, Abdulaziz A, Kabel K, Noah AZ, Youssef A, Dahab AS (2022) Synthesis and Evaluation of Poly (isobutylene-alt-maleic anhydride)-co-Poly (ethylene glycol) and its Copper Oxide Nanocomposite for Enhancing the Performance of Water-based Drilling Fluids in HPHT Wells. Egypt J Chem 65(9):2–4
Elwakeel KZ, El-Liethy MA, Ahmed MS, Ezzat SM, Kamel MM (2018) Facile synthesis of magnetic disinfectant immobilized with silver ions for water pathogenic microorganism’s deactivation. Environ Sci Pollut Res 25(23):22797–22809
Mosmann T (1983) Rapid colorimetric assays for cellular growth and survival : Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Zhang Z, Li J, Ma L, Yang X, Fei B, Leung PHM, Tao X (2020) Mechanistic Study of Synergistic Antimicrobial Effects between Poly (3-hydroxybutyrate) Oligomer and Polyethylene Glycol. Polymers 12:2735
Rekhi P, Goswami M, Ramakrishna S, Debnath M (2022) Polyhydroxyalkanoates biopolymers toward decarbonizing economy and sustainable future. Crit Rev Biotechnol 42(5):668–692
Serini S, Cassano R, Facchinetti E, Amendola G, Trombino S, Calviello G (2019) Anti-irritant and anti-inflammatory effects of DHA encapsulated in resveratrol-based solid lipid nanoparticles in human keratinocytes. Nutrients 11(6):1400
Marichelvam MK, Jawaid M, Asim M (2019) Corn and rice starch-based bio-plastics as alternative packaging materials. Fibers 7(4):32
Acknowledgements
The authors deeply thank the funding organization “The Academy of Scientific Research and Technology (ASRT)” Egypt, for funding and supporting this work through the project entitled “Evaluation and upgrading of the stabilization ponds in Egypt for wastewater reuse, combined with production of value-added bioactive compounds from algae” project ID No.: 4504.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Sayeda M. Abdo: investigation, conceptualization, methodology, writing—original draft. Ahmed M. Youssef: conceptualization, date curation, writing—original draft. Mohamed Azab El-Liethy: Antibacterial analysis, data curation. Gamila H. Ali: formal analysis, data curation, and supervission.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdo, S.M., Youssef, A.M., El-Liethy, M.A. et al. Preparation of simple biodegradable, nontoxic, and antimicrobial PHB/PU/CuO bionanocomposites for safely use as bioplastic material packaging. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-022-03591-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03591-x