Abstract
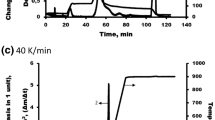
The decrystallization or hydrolysis of lignocellulosic biomass is usually carried out either with concentrated solutions at moderate temperature or with dilute solutions at high temperatures. In contrast to this, agricultural waste biomasses (sunflower stalk, rapeseed stalk, and rice hull) were treated with dilute acidic or alkaline aqueous solutions (5 mol %) in this study to test the variations in cellulose crystallinity under ambient temperature. Solutions of HCl, H3PO4, CH3COOH, HNO3, H2SO4, HF, NaOH, Ca(OH)2, C2H5OH, and CS(NH2)2 were used. Effects of the treatment on cellulose crystallinity were evaluated based on the crystallinity index (CrI) calculations through the reflection intensities in X-ray diffraction (XRD) and the absorbance ratios in Fourier transform-infrared (FTIR) spectroscopy at A1429/A897 (lateral order index) and A1374/A2900 (total crystallinity index). It was found that the CrI values based on the total crystallinity index suited more than lateral order index to the CrI values found by XRD method. HF solutions led to most striking decreases in CrI, while the solutions of neither strong acids nor NaOH resulted in reductions in CrI. Derivative thermogravimetry (DTG) and differential scanning calorimetry (DSC) profiles revealed that the applied treatment influenced the pyrolytic degradation characteristics and the reactivity of biomass in range of 300–400 °C where cellulose decomposed.
Graphical abstract










Similar content being viewed by others
Data availabilty
Data will be made available on reasonable request.
Code availability
Not applicable.
Abbreviations
- ASTM:
-
American Society for Testing and Materials
- CrI:
-
Crystallinity index
- DSC:
-
Differential scanning calorimetry
- DTG:
-
Derivative thermogravimetry
- FTIR:
-
Fourier transform infrared
- HHV:
-
Higher heating value (MJ/kg)
- Ka:
-
Acid dissociation constant
- LOI:
-
Lateral order index
- LHV:
-
Lower heating value (MJ/kg)
- NMR:
-
Nuclear magnetic resonance
- RS:
-
Rapeseed stalk
- RH:
-
Rice hull
- SS:
-
Sunflower stalk
- TCI:
-
Total crystallinity index
- XRD:
-
X-ray diffraction
References
Hoang AT, Ong HC, Fattah IMR, Chong CT, Cheng CK, Sakthivel R, Ok YS (2021) Progress on the lignocellulosic biomass pyrolysis for biofuel production toward environmental sustainability. Fuel Process Technol 223:106997. https://doi.org/10.1016/j.fuproc.2021.106997
Haykiri-Acma H, Yaman S (2022) Effects of torrefaction after pelleting (TAP) process on strength and fuel characteristics of binderless bio‑pellets. Biomass Convers Biorefine 1007/s13399-022-02599-7
Gao C, Yang J, Zhang H, Xiao W, Han L (2020) Quantitative and qualitative characterization of dual scale mechanical enhancement on cellulosic and crystalline-structural variation of Na OH treated wheat straw. Bioresour Technol 312:123535. https://doi.org/10.1016/j.biortech.2020.123535
Poletto M, Zattera AJ, Forte MMC, Santana RMC (2012) Thermal decomposition of wood: influence of wood components and cellulose crystallite size. Bioresour Technol 109:148–153. https://doi.org/10.1016/j.biortech.2011.11.122
Agarwal UP, Ralph SA, Reiner RS, Baez C (2018) New cellulose crystallinity estimation method that differentiates between organized and crystalline phases. Carbohydr Polym 190:262–270. https://doi.org/10.1016/j.carbpol.2018.03.003
Ju X, Bowden M, Brown E, Zhang X (2015) An improved X-ray diffraction method for cellulose crystallinity measurement. Carbohydr Polym 123:476–481. https://doi.org/10.1016/j.carbpol.2014.12.071
Chen H, Liu Z, Chen X, Chen Y, Dong Z, Wang X, Yang H (2020) Comparative pyrolysis behaviors of stalk, wood and shell biomass: correlation of cellulose crystallinity and reaction kinetics. Bioresour Technol 310:123498. https://doi.org/10.1016/j.biortech.2020.123498
Crooker M (2010) Thermochemical conversion of biomass to liquid fuels and chemicals. RSC Publishing, London. ISBN-13: 978-1849730358
Song P, Zhou F, Li F, Han Z, Wang L, Xu J, Zhang B, Wang M, Fan J, Zhang B (2021) Superfine pulverisation pretreatment to enhance crystallinity of cellulose from Lycium barbarum L. leaves. Carbohydr Polym 253:117207. https://doi.org/10.1016/j.carbpol.2020.117207
Zhao D, Yang F, Dai Y, Tao F, Shen Y, Duan W, Zhou X, Ma H, Tang L, Li J (2017) Exploring crystalline structural variations of cellulose during pulpbeating of tobacco stems. Carbohydr Polym 174:146–153. https://doi.org/10.1016/j.carbpol.2017.06.060
Caliari IP, Barbosa MHP, Ferreira SO, Teofilo RF (2017) Estimation of cellulose crystallinity of sugarcane biomass using nearinfrared spectroscopy and multivariate analysis methods. Carbohydr Polym 158:20–28. https://doi.org/10.1016/j.carbpol.2016.12.005
Okon KE, Lin F, Chen Y, Huang B (2017) Effect of silicone oil heat treatment on the chemical composition, cellulose crystalline structure and contact angle of Chinese parasolwood. Carbohydr Polym 164:179–185. https://doi.org/10.1016/j.carbpol.2017.01.076
Wang Z, McDonald AG, Westerhof RJM, Kersten SRA, Cuba-Torres CM, Ha S, Pecha B, Garcia-Perez M (2013) Effect of cellulose crystallinity on the formation of a liquid intermediate and on product distribution during pyrolysis. J Anal Appl Pyrolysis 100:56–66. https://doi.org/10.1016/j.jaap.2012.11.017
Hoang AT, Nizetic S, Ong HC, Mofijur M, Ahmed SF, Ashok B, Bui VTV, Chau MQ (2021) Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere 281:130878. https://doi.org/10.1016/j.chemosphere.2021.130878
Hoang AT, Nizetic S, Ong HC, Chong CT, Atabani AE, Pham VV (2021) Acid-based lignocellulosic biomass biorefinery for bioenergy production: advantages, application constraints, and perspectives. J Env Manage 296:113194. https://doi.org/10.1016/j.jenvman.2021.113194
Zhang L, Loh KC, Zhang J (2018) Food waste enhanced anaerobic digestion of biologically pretreated yard waste: analysis of cellulose crystallinity and microbial communities. Waste Manage 79:109–119. https://doi.org/10.1016/j.wasman.2018.07.036
Yin J, Yuan T, Lu Y, Song K, Li H, Zhao G, Yin Y (2017) Effect of compression combined with steam treatment on the porosity, chemical compositon and cellulose crystalline structure of wood cell walls. Carbohydr Polym 155:163–172. https://doi.org/10.1016/j.carbpol.2016.08.013
Zhang J, Zhang J, Lin L, Chen T, Zhang J, Liu S, Li Z, Ouyang P (2009) Dissolution of microcrystalline cellulose in phosphoric acid— molecular changes and kinetics. Molecules 14:5027–5041. https://doi.org/10.3390/molecules14125027
Chundawat SPS, Agarwal U (2019) Swelling by hydrochloric acid partially retains cellulose-I type allomorphic ultrastructure but enhances susceptibility toward cellulase hydrolysis such as highly amorphous cellulose. In Book: Smith MD (Ed.) Understanding lignocellulosic synergistic computational and analytic methods. ACS Symposium Series. ACS Publications, Washington, DC: pp 69–88
Huntley CJ, Crews KD, Abdala MA, Russell AE, Curry ML (2015) Influence of strong acid hydrolysis processing on the thermal stability and crystallinity of cellulose isolated from wheat straw. Int J Chem Eng 658163. https://doi.org/10.1155/2015/658163
Jin Y, Liu J, Yang H, Shi Z, Zhao P, Yang J (2021) Improving enzymatic saccharification and ethanol production of bamboo residues with sulfomethylation-aided phosphoric acid pretreatment. Ind Crops Prod 170:113733. https://doi.org/10.1016/j.indcrop.2021.113733
Zhang J, Wang Y, Zhang L, Zhang R, Liu G, Cheng G (2014) Understanding changes in cellulose crystalline structure of lignocellulosic biomass during ionic liquid pretreatment by XRD. Bioresour Technol 151:402–405. https://doi.org/10.1016/j.biortech.2013.10.009
Goto M, Yokoe Y (1996) Ammoniation of barley straw. Effect on cellulose crystallinity and water-holding capacity. Anim Feed Sci Technol 58:239–247. https://doi.org/10.1016/0377-8401(95)00903-5
Zhao H, Kwak JH, Wang Y, Franz JA, White JM, Holladay JE (2006) Effects of crystallinity on dilute acid hydrolysis of cellulose by cellulose ball-milling study. Energy Fuels 20:807–811. https://doi.org/10.1021/ef050319a
Satari B, Karimi K, Kumar R (2019) Cellulose solvent-based pretreatment for enhanced second-generation biofuel production: a review. Sustaianble Energy Fuels 3:11–62. https://doi.org/10.1039/C8SE00287H
Kuthi FABA, Badri KH (2014) Effect of cooking temperature on the crystallinity of acid hydrolysed-oil palm cellulose. AIP Conf Proc 1614:456–462. https://doi.org/10.1063/1.4895240
Kanchanalai P, Temani G, Kawajiri Y, Realff MJ (2016) Reaction kinetics of concentrated-acid hydrolysis for cellulose and hemicellulose and effect of crystallinity. BioResources 11:1672–1689. https://doi.org/10.15376/biores.11.1.1672-1689
Chen WH, Nizetic S, Sirohi R, Huang Z, Luque R, Agis M et al (2022) Liquid hot water as sustainable biomass pretreatment technique for bioenergy production: a review. Bioresour Technol 344:126207. https://doi.org/10.1016/j.biortech.2021.126207
Wise LE, Murphy M, D’Addiecs AA (1946) Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Paper Trade J 122:11–19
van Soest PJ (1963) Use of detergents in the analysis of fibrous feeds-II: a rapid method for the determination of fiber and lignin. J Assoc Off Anal Chem 46:829–835
Krässig HA (1993) Cellulose: structure, accessibility and reactivity. Gordon and Breach Science Publ, Philadelphia
Grimaldi MP, Marques MP, Laluce C, Cilli EM, Sponchiado SRP (2015) Evaluation of lime and hydrothermal pretreatments for efficient enzymatic hydrolysis of raw sugarcane bagasse. Biotechnol Biofuels 8:205. https://doi.org/10.1186/s13068-015-0384-y
Siripong P, Duangporn P, Takata E, Tsutsumi Y (2016) Phosphoric acid pretreatment of achyranthes aspera and sida acuta weed biomass to improve enzymatic hydrolysis. Bioresour Technol 203:303–308. https://doi.org/10.1016/j.biortech.2015.12.037
Haykiri-Acma H, Yaman S (2009) Thermogravimetric investigation on the thermal reactivity of biomass during slow pyrolysis. Int J Green Energy 6:333–342. https://doi.org/10.1080/15435070903106959
Klass DL (1998) Biomass for renewable energy, fuels, and chemicals. San Diego: Academic Press. ISBN: 9780124109506
Haykiri-Acma H, Yaman S, Kucukbayrak S (2015) Does carbonization avoid segregation of biomass and lignite during co-firing? Thermal analysis study. Fuel Process Technol 137:312–319. https://doi.org/10.1016/j.fuproc.2015.03.017
Haykiri-Acma H, Yaman S (2008) Thermal reactivity of rapeseed (Brassica napus L.) under different gas atmospheres. Bioresour Technol 99:237–242. https://doi.org/10.1016/j.biortech.2007.01.001
Filho GR, de Assunçao RMN, Vieira JG, Meireles CDS, Cerqueira DA, Barud HDS, Ribeiro SJL, Messaddeq Y (2007) Characterization of methylcellulose produced from sugar cane bagasse cellulose: crystallinity and thermal properties. Polym Degrad Stab 92:205–210. https://doi.org/10.1016/j.polymdegradstab.2006.11.008
Tao X, Li J, Zhang P, Nabi M, Jin S et al (2017) Reinforced acid-pretreatment of Triarrhena lutarioriparia to accelerate its enzymatic hydrolysis. Int J Hydrogen Energy 42:18301–18308. https://doi.org/10.1016/j.ijhydene.2017.04.149
Emam AA, Faraha SAA, Kamal FH, Gamal AM, Basseem M (2020) Modification and characterization of nano cellulose crystalline from Eichhornia crassipes using citric acid: an adsorption study. Carbohydr Polym 240:116202. https://doi.org/10.1016/j.carbpol.2020.116202
Ruan D, Zhang L, Zhou J, Jin H, Chen H (2004) Structure and properties of novel fibers spun from cellulose in NaOH/thiourea aqueous solution. Macromol Biosci 4:1105–1112. https://doi.org/10.1002/mabi.200400120
Zhai R, Chi F, Zhou X (2016) NaOH-Thiourea aqueous solution treatment of cellulose fiber and its effects on bulk and softness. BioResources 11:8703–8719. https://doi.org/10.15376/biores.11.4.8703-8719
Belgacem MN, Pizzi A (2016) Lignocellulosic fibers and wood handbook – renewable materials for today’s environment. Scrivener Publishing, Massachusetts, ISBN 978-1-118-77352-9
Aisiyah MM, Masruri M, Srihardyastutie A (2020) Crystallinity of nanocellulose isolated from the flower waste of pine tree (Pinus merkusii) The 2nd Int Conf on Chemistry and Material Science (IC2MS). IOP Conf. Series: Mate Sci Eng 833:12003. https://doi.org/10.1088/1757-899X/833/1/012003
Wei S, Kumar V, Banker GS (1996) Phosphoric acid mediated depolymerization and decrystallization of cellulose: preparation of low crystallinity cellulose - a new pharmaceutical excipient. Int J Pharm 142:175–181. https://doi.org/10.1016/0378-5173(96)04673-X
Zhang X, Qu T, Mosier NS, Han L, Xiao W (2018) Cellulose modifcation by recyclable swelling solvents. Biotechnol Biofuels 11:191. https://doi.org/10.1186/s13068-018-1191-z
Boerstoel H, Haatman H, Westerink JB, Koenders BM (2001) Liquid crystalline solutions of cellulose in phosphoric acid. Polymer 42:7371–7379. https://doi.org/10.1016/S0032-3861(01)00210-5
Haykiri-Acma H, Yaman S (2019) Effects of dilute phosphoric acid treatment on structure and burning characteristics of lignocellulosic biomass. J Energy Res Technol 141:082203. https://doi.org/10.1115/1.4042719
Park S, Baker JO, Himmel ME, Parilla PA, Johnson DK (2010) Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnol Biofuels 3(1):10. https://doi.org/10.1186/1754-6834-3-10
Long Y, Yu Y, Chua YW, Wu H (2017) Acid-catalysed cellulose pyrolysis at low temperatures. Fuel 193:460–466. https://doi.org/10.1016/j.fuel.2016.12.067
Haykiri-Acma H, Yaman S, Kucukbayrak S (2010) Comparison of the thermal reactivities of isolated lignin and holocellulose during pyrolysis. Fuel Process Technol 91:759–764. https://doi.org/10.1016/j.fuproc.2010.02.009
Cao B, Wang S, Hu Y, Abomohra AEF, Qian L et al (2019) Effect of washing with diluted acids on Enteromorpha clathrata pyrolysis products: towards enhanced bio-oil from seaweeds. Renew Energy 138:29–38. https://doi.org/10.1016/j.renene.2019.01.084
Mohammed IY, Abakr YA, Kazi FK, Yusuf S (2017) Effects of pretreatments of napier grass with deionized water, sulfuric acid and sodium hydroxide on pyrolysis oil characteristics. Waste Biomass Valor 8:755–773. https://doi.org/10.1007/s12649-016-9594-1
David GF, Perez VH, Rodriguez Justo O, Garcia-Perez M (2017) Effect of acid additives on sugarcane bagasse pyrolysis: production of high yields of sugars. Bioresour Technol 223:74–83. https://doi.org/10.1016/j.biortech.2016.10.051
Author information
Authors and Affiliations
Contributions
Equal.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
> Effects of dilute solutions on cellulose crystallinity were studied comparatively.
> The solution of HF is the most efficient in terms of reduction in cellulose crystallinity.
> Compared to A1429/A897 (lateral order index), CrI values based on A1374/A2900 (total crystallinity index) are more consistent with those found by XRD.
> Applied treatment affected the pyrolytic degradation characteristics at temperatures between 300 and 400 °C where cellulose decomposed.
Statement of novelty
Insufficient attention has been paid to the treatment of lignocellulosic biomass using dilute solutions under ambient temperature, as it appears to be ineffective. In this study, it was aimed to determine how the cellulose crystallinity of lignocellulosic biomass treated by dilute solutions at ambient temperature is affected. In addition, a comparative evaluation was made to determine to what extent the cellulose crystallinity index (CrI) values determined according to XRD and FTIR techniques were compatible with each other. Also, effects of applied treatment on the pyrolytic degradation and the reactivity of cellulose were also studied. The results showed that the treatment with dilute solutions can have a non-negligible effect on the cellulose crystallinity of the lignocellulosic biomass, contrary to expectations.
Rights and permissions
About this article
Cite this article
H., HA., S., Y. Treating lignocellulosic biomass with dilute solutions at ambient temperature: effects on cellulose crystallinity. Biomass Conv. Bioref. 14, 9967–9981 (2024). https://doi.org/10.1007/s13399-022-03085-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03085-w