Abstract
The expanding urbanization and industrialization lead to a large amount of wastewater discharge that is toxic and harmful to the environment. In particular, the discharge from dairy industries has chemical oxygen demand (COD) up to 95,000 mg/L and other pollutants that exceed the standard limit and are hazardous. Wastewater can be treated efficiently using microalgae which reduces the pollutants and also yields higher biomass that is further processed to produce various value-added products. This study investigated the effect of Chlorella vulgaris in treating wastewater collected from the dairy industry. Three different growth mediums, such as modified CHU-10, Bold Basal, and BG-11 (Blue Green-11), were tested for algal growth. Since the turbidity of wastewater was high, the dairy wastewater was diluted with distilled water at various concentrations of 100% (pure), 75%, 50%, and 25%. Chlorella vulgaris showed a promising result when grown in dairy wastewater, with a maximum biomass concentration of 2.43 g/L and maximum biomass productivity of 225 g/L/day. Overall, the bioremediation results revealed that the maximum removal efficiency was 81.48% (COD), 87.70% (total nitrogen-TN), and 93.5% (total phosphorus-TP). The lipid composition consists largely of c16:0 (hexadecanoic acid), c18:2 (linoleic acid), and c18:3 (linolenic acid), which shows the obtained FAME (fatty acid methyl esters) has suitable properties for biodiesel production. The Michaelis–Menten model was used for the batch kinetic studies, the kinetic coefficients of total nitrogen, and total phosphorus removal were determined for total nitrogen kinetic coefficients (km) = 198.77 mg/L, saturation constant (k) = 0.025 mg TN/mg biomass day, and R2 = 0.9547 and for total phosphorus removal as km = 668.09 mg/L, k = 0.17 mg TP/mg biomass day, and R2 = 0.9947. These results showed that the C. vulgaris is a suitable microalga that can grow in high turbidity DWW resulting in pollutant removal, higher biomass, and reducing the environmental impact caused by wastewater.
Graphical abstract











Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Ji X, Li H, Zhang J et al (2019) The collaborative effect of Chlorella vulgaris-Bacillus licheniformis consortia on the treatment of municipal water. J Hazard Mater 365:483–493. https://doi.org/10.1016/j.jhazmat.2018.11.039
Lewkowska P, Cieślik B, Dymerski T et al (2016) Characteristics of odors emitted from municipal wastewater treatment plant and methods for their identification and deodorization techniques. Environ Res 151:573–586
Gupta VK, Ali I (2013) Wastewater treatment by biological methods. Environ Water 179–204. https://doi.org/10.1016/b978-0-444-59399-3.00007-6
Zhang R, Xie WM, Yu HQ, Li WW (2014) Optimizing municipal wastewater treatment plants using an improved multi-objective optimization method. Bioresour Technol 157:161–165. https://doi.org/10.1016/j.biortech.2014.01.103
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sustain Energy Rev 19:360–369
Basiri Parsa J, Hagh Negahdar S (2012) Treatment of wastewater containing Acid Blue 92 dye by advanced ozone-based oxidation methods. Sep Purif Technol 98:315–320. https://doi.org/10.1016/j.seppur.2012.06.041
Su-xia H, Ji-jun L, Bin H et al (2014) The treatment of radioactive wastewater by ultrasonic standing wave method. J Hazard Mater 274:41–45. https://doi.org/10.1016/j.jhazmat.2014.03.068
Wang Y, Guo W, Yen HW et al (2015) Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour Technol 198:619–625. https://doi.org/10.1016/j.biortech.2015.09.067
Ahmad A, Banat F, Alsafar H, Hasan SW (2022) Algae biotechnology for industrial wastewater treatment, bioenergy production, and high-value bioproducts. Sci Total Environ 806:150585. https://doi.org/10.1016/j.scitotenv.2021.150585
Wang K, Brown RC, Homsy S et al (2013) Fast pyrolysis of microalgae remnants in a fluidized bed reactor for bio-oil and biochar production. Bioresour Technol 127:494–499. https://doi.org/10.1016/j.biortech.2012.08.016
González LE, Cañizares RO, Baena S (1997) Efficiency of ammonia and phosphorus removal from a colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour Technol 60:259–262. https://doi.org/10.1016/S0960-8524(97)00029-1
Jain D, Ghonse SS, Trivedi T et al (2019) CO 2 fixation and production of biodiesel by Chlorella vulgaris NIOCCV under mixotrophic cultivation. Bioresour Technol 273:672–676. https://doi.org/10.1016/j.biortech.2018.09.148
Fu W, Gudmundsson S, Wichuk K et al (2019) Sugar-stimulated CO 2 sequestration by the green microalga Chlorella vulgaris. Sci Total Environ 654:275–283. https://doi.org/10.1016/j.scitotenv.2018.11.120
Chu F, Cheng J, Zhang X et al (2019) Enhancing lipid production in microalgae Chlorella PY-ZU1 with phosphorus excess and nitrogen starvation under 15% CO2 in a continuous two-step cultivation process. Chem Eng J 375:121912. https://doi.org/10.1016/j.cej.2019.121912
Chiu S, Kao C, Chen T et al (2015) Bioresource technology cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour Technol 184:179–189. https://doi.org/10.1016/j.biortech.2014.11.080
Rude K, Yothers C, Barzee TJ et al (2022) Growth potential of microalgae on ammonia-rich anaerobic digester effluent for wastewater remediation. Algal Res 62:102613. https://doi.org/10.1016/j.algal.2021.102613
Wu YH, Hu HY, Yu Y et al (2014) Microalgal species for sustainable biomass/lipid production using wastewater as resource: a review. Renew Sustain Energy Rev 33:675–688
Sukla LB, Pradhan D, Subbaiah T (2019) Future prospects of microalgae in wastewater treatment. Role Microalgae Wastewater Treat 129–135. https://doi.org/10.1007/978-981-13-1586-2_10
Li H, Zhang Y, Liu J et al (2019) Treatment of high-nitrate wastewater mixtures from MnO2 industry by Chlorella vulgaris. Bioresour Technol 291:121836. https://doi.org/10.1016/j.biortech.2019.121836
Yeh KL, Chang JS (2012) Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour Technol 105:120–127. https://doi.org/10.1016/j.biortech.2011.11.103
Church J, Hwang JH, Kim KT et al (2017) Effect of salt type and concentration on the growth and lipid content of Chlorella vulgaris in synthetic saline wastewater for biofuel production. Bioresour Technol 243:147–153. https://doi.org/10.1016/j.biortech.2017.06.081
Hung Y-T, Britz T, van Schalkwyk C (2005) Treatment of dairy processing wastewaters. Waste Treat Food Process Ind 1–28. https://doi.org/10.1201/9781420037128.ch1
Gao K, Liu Q, Gao Z et al (2021) A dilution strategy used to enhance nutrient removal and biomass production of Chlorella sorokiniana in frigon wastewater. Algal Res 58:102438. https://doi.org/10.1016/j.algal.2021.102438
Mahmood HM, Abed IJ, Al-Mashhadani MKH (2017) Evaluating harvesting of Chlorella sp. biomass and chemical composition under the influence of different concentrations of nutrients. Curr Res Microbiol Biotechnol 5:1375–1379
2012 A 1967 Future of standard methods for the examination of water and wastewater
Ilavarasi A, Mubarakali D, Praveenkumar R et al (2011) Optimization of various growth media to freshwater microalgae for biomass production. Biotechnology 10:540–545. https://doi.org/10.3923/biotech.2011.540.545
Ramírez-López C, Chairez I, Fernández-Linares L (2016) A novel culture medium designed for the simultaneous enhancement of biomass and lipid production by Chlorella vulgaris UTEX 26. Bioresour Technol 212:207–216. https://doi.org/10.1016/J.BIORTECH.2016.04.051
Bligh, E.G. and Dyer WJ 1959 Canadian Journal of Biochemistry and Physiology. Can J Biochem Physiol 37
Trivedi T, Jain D, Mulla NSS et al (2019) Improvement in biomass, lipid production and biodiesel properties of a euryhaline Chlorella vulgaris NIOCCV on mixotrophic cultivation in wastewater from a fish processing plant. Renew Energy 139:326–335. https://doi.org/10.1016/j.renene.2019.02.065
Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng 28:64–70. https://doi.org/10.1016/j.ecoleng.2006.04.003
El-Sheekh MM, Morsi HH, Hassan LHS (2020) Assessment of the optimum growth medium and the effect of different light intensities on growth and photosynthetic pigments of chlorella vulgaris and scenedesmus arvernensis. Egypt J Bot 60:395–404. https://doi.org/10.21608/ejbo.2019.18274.1367
Tao R, Kinnunen V, Praveenkumar R et al (2017) Comparison of Scenedesmus acuminatus and Chlorella vulgaris cultivation in liquid digestates from anaerobic digestion of pulp and paper industry and municipal wastewater treatment sludge. J Appl Phycol 29:2845–2856. https://doi.org/10.1007/s10811-017-1175-6
Junping Lv, Yang Liu, Jia Fen,g Qi Liu, Fangru Nan, Shulian Xie 2018 Nutrients removal from undiluted cattle farm wastewater by the two-stage process of microalgae-based wastewater treatmenttle. Bioresour Technol
Hamidian N, Zamani H (2022) Biomass production and nutritional properties of Chlorella sorokiniana grown on dairy wastewater. J Water Process Eng 47:102760. https://doi.org/10.1016/J.JWPE.2022.102760
Gupta S, Pawar SB, Pandey RA (2019) Current practices and challenges in using microalgae for treatment of nutrient rich wastewater from agro-based industries. Sci Total Environ 687:1107–1126
Cho HJ (2016) Dairy wastewater treatment using microalgae for potential biodiesel application. Environ Eng Res 21:393–400. https://doi.org/10.4491/eer.2015.151
Tolfo J, Sebastião G, Farenzena M, Gutterres M (2017) Influence of light intensity and tannery wastewater concentration on biomass production and nutrient removal by microalgae Scenedesmus sp. Process Saf Environ Prot 111:355–362. https://doi.org/10.1016/j.psep.2017.07.024
Škufca D, Kovačič A, Prosenc F et al (2021) Phycoremediation of municipal wastewater: removal of nutrients and contaminants of emerging concern. Sci Total Environ 782:146949. https://doi.org/10.1016/J.SCITOTENV.2021.146949
Pathak VV, Singh DP, Kothari R, Chopra AK (2014) Phycoremediation of textile wastewater by unicellular microalga Chlorella pyrenoidosa. Cell Mol Biol 60:35–40. https://doi.org/10.14715/cmb/2014.60.5.7
Pena ACC, Agustini CB, Trierweiler LF (2020) Influence of period light on cultivation of microalgae consortium for the treatment of tannery wastewaters from leather fi nishing stage. J Clean Prod 263:121618. https://doi.org/10.1016/j.jclepro.2020.121618
Daneshvar E, Antikainen L, Koutra E et al (2018) Investigation on the feasibility of Chlorella vulgaris cultivation in a mixture of pulp and aquaculture effluents: treatment of wastewater and lipid extraction. Bioresour Technol 255:104–110. https://doi.org/10.1016/j.biortech.2018.01.101
Widjaja A, Chien CC, Ju YH (2009) Study of increasing lipid production from fresh water microalgae Chlorella vulgaris. J Taiwan Inst Chem Eng 40:13–20. https://doi.org/10.1016/J.JTICE.2008.07.007
Shahid A, Malik S, Zhu H et al (2020) Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci Total Environ 704:135303. https://doi.org/10.1016/j.scitotenv.2019.135303
Zhou W, Li Y, Min M et al (2011) Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresour Technol 102:6909–6919. https://doi.org/10.1016/J.BIORTECH.2011.04.038
Qin L, Wang Z, Sun Y et al (2016) Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ Sci Pollut Res 23:8379–8387. https://doi.org/10.1007/s11356-015-6004-3
Evans L, Hennige SJ, Willoughby N et al (2017) Effect of organic carbon enrichment on the treatment efficiency of primary settled wastewater by Chlorella vulgaris. Algal Res 24:368–377. https://doi.org/10.1016/j.algal.2017.04.011
Pandey P, Shi J (2017) Assessing nutrient removal kinetics in flushed manure using Chlorella vulgaris biomass production. Front Bioeng Biotechnol 5. https://doi.org/10.3389/fbioe.2017.00043
Author information
Authors and Affiliations
Contributions
Sudhanthiran M. C.: conceptualization, methodology, data curation, writing—original draft preparation, writing—reviewing, and editing
Muthiah Perumalsamy: conceptualization, methodology, supervision, writing—reviewing, and editing
Corresponding author
Ethics declarations
This study does not involve any human subjects.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Phycoremediation of dairy Industry wastewater (DWW) using Chlorella vulgaris was studied.
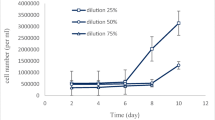
• High Chlorella vulgaris biomass growth was observed in diluted dairy industry wastewater: 2.43 g/L.
• The maximum removal efficiency was observed in 75% diluted dairy industry wastewater: 81.48% (COD), 87.70% (total nitrogen-TN), and 93.5% (total phosphorus-TP).
• The major fatty acids, such as C16:0 (hexadecanoic acid), C18:2 (linoleic acid), and C18:3 (linolenic acid), were present in the obtained lipids, which constitute 88% of total FAME.
Rights and permissions
About this article
Cite this article
C., S.M., Perumalsamy, M. Bioremediation of dairy industry wastewater and assessment of nutrient removal potential of Chlorella vulgaris. Biomass Conv. Bioref. 14, 10335–10346 (2024). https://doi.org/10.1007/s13399-022-03068-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03068-x