Abstract
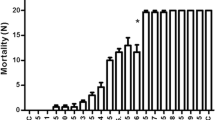
Dengue is a mosquito-borne viral disease occurring in many countries and the seaweeds are considered to be the best sources of antiviral compounds. Present study focuses on the toxicity potential of the largely untapped brown and red seaweeds-extracts viz; Sargassum wightii & Gelidiella acerosa, vis-à-vis dengue causing, Aedes aegypti. The pupicidal activity of the ethyl acetate seaweed—extracts revealed highest mortality rate. The S. wightii—ethyl acetate (SW-EA) caused the increased toll of Aedes aegypti—pupae with the lethal (LC-50 & LC-90) doses (μg/mL) of; 12.83 & 50.83 respectively and the G. acerosa- ethyl acetate (GA-EA) caused the increased death-toll of Aedes aegypti—pupae showing the lethality values of 17.66 (LC50) & 55.71 (LC90). And the histopathological studies showed the malformations (midgut, gut lumen, muscles and epithelial layer) in the bodies of the mosquito pupae treated by crude extracts of SW-EA & GA-EA. Although some reports are already available on the mosquito-larvicidal property of seaweeds, the present research-findings form the first report on the high-mosquito-pupicidal value and cytotoxicity in the dengue vector, Ae. aegypti. The generated data underlines the importance of these seaweeds as potential mosquitocidal agents and further-more research is needed in future.

Graphical Abstract




Similar content being viewed by others
References
Benelli G (2015) Research in mosquito control: current challenges for a brighter future. Parasitol Res. 114(8):2801–2805. https://doi.org/10.1007/s00436-015-4586-9
WHO, World Malaria Report (2020) World Health Organization. Switzerland, Geneva. https://cdn.who.int/media/docs/default-source/malaria/world-malaria-reports/9789240015791-double-page-view.pdf?sfvrsn=2c24349d_10
Senthilkumar A, Kannathasan K, Venkatesalu V (2008) Chemical constituents and larvicidal property of the essential oil of Blumea mollis (D. Don) Merr. against Culex quinquefasciatus. Parasitol Res 103(4):959–962. https://doi.org/10.1007/s00436-008-1085-2
Beula JM, Ravikumar S, Ali MS (2011) Mosquito larvicidal efficacy of seaweed extracts against dengue vector of Aedes aegypti. Asian Pac J Trop Biomed 1(2):S143–S146. https://doi.org/10.1016/S2221-1691(11)60143-3
Brown AW (1986) Insecticide resistance in mosquitoes: a pragmatic review. J Am Mosq Control Assoc 2(2):123–140. https://europepmc.org/article/med/2906965
Russell TL, Kay BH, Skilleter GA (2009) Environmental effects of mosquito insecticides on saltmarsh invertebrate fauna. Aquat Biol 6:77–90. https://doi.org/10.3354/ab00156
Kumar KP, Murugan K, Kovendan K, Kumar AN, Hwang JS, Barnard DR (2012) Combined effect of seaweed (Sargassum wightii) and Bacillus thuringiensis var. israelensis on the coastal mosquito, Anopheles sundaicus. Tamil Nadu, India. Sci Asia 38:141–146. https://doi.org/10.2306/scienceasia1513-1874.2012.38.141
Kalimuthu K, Lin SM, Tseng LC, Murugan K, Hwang JS (2014) Bio-efficacy potential of seaweed Gracilaria firma with copepod, Megacyclops formosanus for the control larvae of dengue vector Aedes aegypti. Hydrobiologia 741(1):113–123. https://doi.org/10.1007/s10750-013-1745-9
Thangam T, Kathiresan K (1991) Mosquito larvicidal effect of seaweed extracts. Bot Mar 34(5):433–436. https://doi.org/10.1515/botm.1991.34.5.433
Mahyoub JA (2018) Mosquito Larvicidal activity of seaweed extracts against Anopheles d’thali with reference to its side effects on aquatic nontarget organisms. Int J Mosq Res 5(6):34–38. https://www.dipterajournal.com/pdf/2018/vol5issue6/PartA/5-5-24-288.pdf
Murugan K, Samidoss CM, Panneerselvam C, Higuchi A, Roni M, Suresh U, Chandramohan B, Subramaniam J, Madhiyazhagan P, Dinesh D, Rajaganesh R (2015) Seaweed-synthesized silver nanoparticles: an eco-friendly tool in the fight against Plasmodium falciparum and its vector Anopheles stephensi? Parasitol Res 114(11):4087–4097. https://doi.org/10.1007/s00436-015-4638-1
Madhiyazhagan P, Murugan K, Kumar AN, Nataraj T, Dinesh D, Panneerselvam C, Subramaniam J, Kumar PM, Suresh U, Roni M, Nicoletti M (2015) Sargassum muticum-synthesized silver nanoparticles: An effective control tool against mosquito vectors and bacterial pathogens. Parasitol Res 114(11):4305–4317. https://doi.org/10.1007/s00436-015-4671-0
Haleem DR, El Tablawy NH, Alkeridis LA, Sayed S, Saad AM, El-Saadony MT, Farag SM (2022) Screening and evaluation of different algal extracts and prospects for controlling the disease vector mosquito Culex pipiens L. Saudi J Biol Sci 29(2):933–940. https://doi.org/10.1016/j.sjbs.2021.10.009
Kokilaramani S, Rajasekar A, AlSalhi MS, Devanesan S (2021) Characterization of methanolic extract of seaweeds as environmentally benign corrosion inhibitors for mild steel corrosion in sodium chloride environment. J Mol Liq 340:117011. https://doi.org/10.1016/j.molliq.2021.117011
Kamaraj C, Bagavan A, Rahuman AA, Zahir AA, Elango G, Pandiyan G (2009) Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae). Parasitol Res 104(5):1163–1171. https://doi.org/10.1007/s00436-008-1306-8
Abbott WS (1925) A method of computing the effectiveness of an insecticide. J econ Entomol 18(2):265–267. https://doi.org/10.1093/jee/18.2.265a
Vivekanandhan P, Venkatesan R, Ramkumar G, Karthi S, Senthil-Nathan S, Shivakumar MS (2018) Comparative analysis of major mosquito vectors response to seed-derived essential oil and seed pod-derived extract from Acacia nilotica. Int J Environ Res and Public Health 15(2):388. https://doi.org/10.1016/j.saa.2014.08.064
Shaalan EA, Canyon DV (2009) Aquatic insect predators and mosquito control. Trop Biomed 26:223–261. https://researchonline.jcu.edu.au/11515/1/11515_Shaalan_%26_Canyon_2009.pdf
Venkadachalam R, Subramaniyan V, Palani M, Subramaniyan M, Srinivasan P, Raji M (2017) Mosquito larvicidal and pupicidal activity of Tephrosia purpurea Linn.(Family: Fabaceae) and Bacillus sphaericus against, dengue vector, Aedes aegypti. Pharmacogn J 9(6):737–742. https://doi.org/10.5530/pj.2017.6.116
Nasir S, Nasir I, Asrar M, Debboun M (2017) Larvicidal and pupicidal action of medicinal plant extracts against dengue mosquito Aedes albopictus (Skuse) (Diptera: Culicidae). Indian J Anim Res 51(1):155–158. https://doi.org/10.18805/ijar.9592
Geetha I, Manonmani AM (2008) Mosquito pupicidal toxin production by Bacillus subtilis subsp. subtilis. Biol Control 44(2):242–247. https://doi.org/10.1016/j.biocontrol.2007.10.007
Foko LP, Meva FE, Moukoko CE, Ntoumba AA, Ekoko WE, Belle PE, Ndjouondo GP, Bunda GW, Lehman LG (2021) Green-synthesized metal nanoparticles for mosquito control: A systematic review about their toxicity on non-target organisms. Acta Trop 214:105792. https://doi.org/10.1016/j.actatropica.2020.105792
Panneerselvam C, Murugan K, Kovendan K, Kumar PM, Subramaniam J (2013) Mosquito larvicidal and pupicidal activity of Euphorbia hirta Linn. (Family: Euphorbiaceae) and Bacillus sphaericus against Anopheles stephensi Liston.(Diptera: Culicidae). Asian Pac J Trop Med 6(2):102–109. https://doi.org/10.1016/S1995-7645(13)60003-6
Hira VS, Tariq RM, Ara J, Ehteshamul-Haque S (2010) Larvicidal activity of marine macro-algae from Karachi coast against dengue virus vector mosquito, the Aedes aegypti L. Pak J Entomol Karachi 25(2):143–146. https://www.researchgate.net/profile/Drhakim-Sahito/publication/260368464_STUDIES_ON_VARIETAL_RESISTANCE_OF_SUNFLOWER_CROP_AGAINST_BEMISIA_TABACI_GENN_AND_AMRASCA_DEVASTANS_DIST_LANJAR_AG_AND_SAHITO_HA/links/00b7d530eed6267dc8000000/STUDIES-ON-VARIETAL-RESISTANCE-OF-SUNFLOWER-CROP-AGAINST-BEMISIA-TABACI-GENN-AND-AMRASCA-DEVASTANS-DIST-LANJAR-AG-AND-SAHITO-HA.pdf#page=84
Ishwarya R, Vaseeharan B, Kalyani S, Banumathi B, Govindarajan M, Alharbi NS, Kadaikunnan S, Al-Anbr MN, Khaled JM, Benelli G (2018) Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J Photochem Photobiol B Biol 178:249–258. https://doi.org/10.1016/j.jphotobiol.2017.11.006
Chantawee A, Soonwera M (2018) Efficacies of four plant essential oils as larvicide, pupicide and oviposition deterrent agents against dengue fever mosquito, Aedes aegypti Linn. (Diptera: Culicidae). Asian Pac J Trop Med 8(4): 217–225. https://doi.org/10.4103/2221-1691.231284
Sivapriyajothi S, Kumar PM, Kovendan K, Subramaniam J, Murugan K (2014) Larvicidal and pupicidal activity of synthesized silver nanoparticles using Leucas aspera leaf extract against mosquito vectors, Aedes aegypti and Anopheles stephensi. J J Entomol Acarol 46(2):77–84. https://doi.org/10.4081/jear.2014.1787
Roni M, Murugan K, Panneerselvam C, Subramaniam J, Nicoletti M, Madhiyazhagan P, Dinesh D, Suresh U, Khater HF, Wei H, Canale A (2015) Characterization and biotoxicity of Hypnea musciformis-synthesized silver nanoparticles as potential eco-friendly control tool against Aedes aegypti and Plutella xylostella. Ecotoxicol Environ Saf 46(2):77–84. https://doi.org/10.1016/j.ecoenv.2015.07.005
Kovendan K, Murugan K, Naresh Kumar A, Vincent S, Hwang JS (2012) Bioefficacy of larvicdial and pupicidal properties of Carica papaya (Caricaceae) leaf extract and bacterial insecticide, spinosad, against chikungunya vector, Aedes aegypti (Diptera: Culicidae). Parasitol Res 110(2):669–678. https://doi.org/10.1007/s00436-011-2540-z
Adaikala Raj G, Jayaraman M, Krishnamoorthy S, Chandrasekaran M, Venkatesalu V, (2017) Screening of different extracts of marine macro green algae for larvicidal activity against dengue fever mosquito, Aedes aegypti (Diptera: Culiadae). Int Lett Nat Sci 62:44–51. https://doi.org/10.18052/www.scipress.com/ILNS.62.44
Panneerselvam C, Murugan K, Kovendan K, Mahesh KP (2012) Mosquito larvicidal, pupicidal, adulticidal, and repellent activity of Artemisia nilagirica (Family: Compositae) against Anopheles stephensi and Aedes aegypti. Parasitol Res 111(6):2241–2251. https://doi.org/10.1007/s00436-012-3073-9
Raveen R, Ahmed F, Pandeeswari M, Reegan D, Tennyson S, Arivoli S, Jayakumar M (2017) Laboratory evaluation of a few plant extracts for their ovicidal, larvicidal and pupicidal activity against medically important human dengue, chikungunya and Zika virus vector, Aedes aegypti Linnaeus 1762 (Diptera: Culicidae). Int J Mosq Res 4(4):17–28. https://www.dipterajournal.com/pdf/2017/vol4issue4/PartA/4-4-8-299.pdf
Murugan K, Roni M, Panneerselvam C, Suresh U, Rajaganesh R, Aruliah R, Mahyoub JA, Trivedi S, Rehman H, Al-Aoh HAN, Kumar S (2018) Sargassum wightii-synthesized ZnO nanoparticles reduce the fitness and reproduction of the malaria vector Anopheles stephensi and cotton bollworm Helicoverpa armigera. Physiol Mol Plant Pathol 101:202–213. https://doi.org/10.1016/j.pmpp.2017.02.004
Abutaha N, Al-mekhlafi FA, Wadaan MA, Al-Khalifa MS (2022) Larvicidal activity and chemical compositions of Aloe ferox mill, and Commipora abyssinica (O. J King Saud Univ Sci, Berg) combination against the mosquito vectors Culex pipiens L. J King Saud Univ Sci 34(4):101962. https://doi.org/10.1016/j.jksus.2022.101962
Syad AN, Rajamohamed BS, Shunmugaiah KP, Kasi PD (2016) Neuroprotective effect of the marine macroalga Gelidiella acerosa: Identification of active compounds through bioactivity-guided fractionation. Pharm Biol 54(10):2073–2081. https://doi.org/10.3109/13880209.2016.1145700
Balachandran P, Parthasarathy V, Kumar TA (2016) Isolation of compounds from Sargassum wightii by GCMS and the molecular docking against anti-inflammatory marker COX2. Int Lett Chem Phys Astron 63:1–12. https://doi.org/10.18052/www.scipress.com/ILCPA.63.1
Sowmiya R, Prasanna Kumar S, Deepak P, Ramkumar R, Balasubramani G, Aiswarya D, Perumal P, Ravikumar S (2016) In vitro antiplasmodial activity of native Indian seaweed Sargassum Sp. Asian J Pharm Clin Res 9(2):101–6. https://innovareacademics.in/journals/index.php/ajpcr/article/view/9935
Sahar AB (2010) Histopathological effects of fenugreek [trigonella foenum-graceum] extracts on the larvae of the mosquito Culex quinquefasciatus. J Arab Society Med Res 5(2):123–130. https://pesquisa.bvsalud.org/portal/resource/pt/emr-117221
Manimaran K, Murugesan S, Ragavendran C, Balasubramani G, Natarajan D, Ganesan A, Seedevi P (2021) Biosynthesis of TiO Nanoparticles using edible mushroom (pleurotus djamor) extract: mosquito larvicidal, histopathological, antibacterial and anticancer effect. J Clust Sci 32(5):1229–1240. https://doi.org/10.1007/s10876-020-01888-3
Bibi R, Tariq RM, Rasheed M (2020) Toxic assessment, growth disrupting and neurotoxic effects of red seaweeds’ botanicals against the dengue vector mosquito Aedes aegypti L. Ecotoxicol Environ Saf 195:110451. https://doi.org/10.1016/j.ecoenv.2020.110451
Acknowledgements
Grateful thanks are due to Prof. R. Arthur James, Head, Department of Marine Science, Bharathidasan University, Tiruchirappalli, for the facility extended, to the Field unit of Vector Control Research Centre (ICMR, Govt. of India), Madurai, for their supply of mosquito-eggs and larvae and to the UGC, New Delhi, for the award of BSR-Faculty Fellowship to PP (Ref. No.F.18-1/2011 (BSR), 26.06.2018).
Author information
Authors and Affiliations
Contributions
Sundaramoorthy Dhanasundaram: Writing – review & editing, Formal analysis, Resources, Data curation. Annamalai Aravinth: Data curation, review, Mapping, Formal analysis. Pachiappan Perumal: Methodology, Investigation, Conceptualization, writing – review & editing, Supervision, Project administration. Vadivel Amutha: Writing – review & editing, statistical analysis. Rajaram Rajendran: Investigation, Conceptualization, writing – review & editing, Supervision, Project administration. Perumal Santhanam: Review & editing, Data curation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhanasundaram, S., Aravinth, A., Perumal, P. et al. Mosquito-pupicidal activity of the extracts of seaweeds, Sargassum wightii, and Gelidiella acerosa against the dengue vector, Aedes aegypti. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-03064-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-03064-1