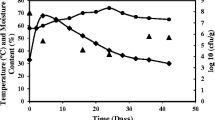
Abstract
In the cold regions, the problem of efficient degradation of agro-residual waste can be overwhelmed by bioprospecting the extremozymes from cold-adapted microorganisms. The compost habitat provides a hostile environment that harbours an enormous group of microbial communities with potential hydrolytic activities. Exploring these microorganisms would be beneficial to avert the crop residual resources to fulfil the future resource demand. In the present study, a cold-adapted bacterium, Exiguobacterium sibiricum K1, with cellulase activity, was isolated from the compost sample of Sikkim, India. Under optimized conditions, the maximum cellulase production achieved was 3.8 U/mL at 15 ℃ and pH 5. The bacterial cold-active cellulase was investigated to degrade four agro-residual wastes, namely sugar cane bagasse, wheat bran, corn stover, and oil-extracted lemongrass. In scanning electron micrographs, the enzyme-treated wastes showed structural breakages in the form of pores and cracks. However, no significant changes were observed in the untreated groups. Furthermore, the hydrolysate in gas chromatography–mass spectrometry showed the release of thirteen products in both sugar cane bagasse and wheat bran enzymolysis. However, twelve products were found in corn stover and fourteen in lemongrass degradation. The products were categorized into alcohol, saccharide, acid, ketone, ester, alkane, aldehyde, and furan. Additionally, the hydrolysate showed the availability of several phytonutrients such as Fe, Mn, Cu, Zn, Ca, and K in enzyme-treated test groups compared to the untreated waste. Hence, applying hydrolysate to soil may help enhance soil quality by providing nutrients essential to plant growth. Thus, microbial intervention could be a better treatment strategy to manage agro-residual waste in cold regions and resource recovery that will contribute to an eco-friendly environment and sustainable bio-economy in the future.
Graphical abstract




Similar content being viewed by others
References
Onwosi CO, Igbokwe VC, Odimba JN et al (2017) Composting technology in waste stabilization: on the methods, challenges and future prospects. J Environ Manage 190:140–157. https://doi.org/10.1016/j.jenvman.2016.12.051
Ramachandra TV, Bharath HA, Kulkarni G, Han SS (2018) Municipal solid waste: generation, composition and GHG emissions in Bangalore, India. Renew Sustain Energy Rev 82:1122–1136. https://doi.org/10.1016/j.rser.2017.09.085
Kumari S, Manyapu V, Kumar R (2022) Recent advances in composting and vermicomposting techniques in the cold region: resource recovery, challenges, and way forward. Adv Org Waste Manag 131–154. https://doi.org/10.1016/B978-0-323-85792-5.00005-8
Dhar H, Kumar S, Kumar R (2017) A review on organic waste to energy systems in India. Bioresour Technol 245:1229–1237. https://doi.org/10.1016/j.biortech.2017.08.159
Bhuvaneshwari S, Hettiarachchi H, Meegoda JN (2019) Crop residue burning in India: Policy challenges and potential solutions. Int J Environ Res Public Health 16:832. https://doi.org/10.3390/ijerph16050832
Gadde B, Bonnet S, Menke C, Garivait S (2009) Air pollutant emissions from rice straw open field burning in India, Thailand and the Philippines. Environ Pollut 157:1554–1558. https://doi.org/10.1016/j.envpol.2009.01.004
Sarsaiya S, Jain A, Kumar Awasthi S et al (2019) Microbial dynamics for lignocellulosic waste bioconversion and its importance with modern circular economy, challenges and future perspectives. Bioresour Technol 291:121905. https://doi.org/10.1016/j.biortech.2019.121905
Jiang C, Cheng Y, Zang H et al (2020) Biodegradation of lignin and the associated degradation pathway by psychrotrophic Arthrobacter sp. C2 from the cold region of China. Cellulose 27:1423–1440. https://doi.org/10.1007/s10570-019-02858-3
Chen P, Anderson E, Addy M et al (2018) Breakthrough technologies for the biorefining of organic solid and liquid wastes. Engineering 4:574–580. https://doi.org/10.1016/j.eng.2018.07.004
Li Y, Han Y, Zhang Y et al (2020) Factors affecting gaseous emissions, maturity, and energy efficiency in composting of livestock manure digestate. Sci Total Environ 731:139157. https://doi.org/10.1016/j.scitotenv.2020.139157
Guerriero G, Hausman JF, Strauss J et al (2016) Lignocellulosic biomass: biosynthesis, degradation, and industrial utilization. Eng Life Sci 16:1–16. https://doi.org/10.1002/elsc.201400196
Gupta SK, Kataki S, Chatterjee S et al (2020) Cold adaptation in bacteria with special focus on cellulase production and its potential application. J Clean Prod 258:120351. https://doi.org/10.1016/j.jclepro.2020.120351
Li D, Feng L, Liu K et al (2016) Optimization of cold-active CMCase production by psychrotrophic Sphingomonas sp. FLX-7 from the cold region of China. Cellulose 23:1335–1347. https://doi.org/10.1007/s10570-016-0859-4
Sun S, Zhang Y, Liu K et al (2020) Insight into biodegradation of cellulose by psychrotrophic bacterium Pseudomonas sp. LKR-1 from the cold region of China: optimization of cold-active cellulase production and the associated degradation pathways. Cellulose 27:315–333. https://doi.org/10.1007/s10570-019-02798-y
Steiner E, Margesin R (2020) Production and partial characterization of a crude cold-active cellulase (CMCase) from Bacillus mycoides AR20-61 isolated from an Alpine forest site. Ann Microbiol 70:67. https://doi.org/10.1186/s13213-020-01607-3
Kumar R, Singh D, Swarnkar MK et al (2015) Complete genome sequence of Arthrobacter sp. ERGS1:01, a putative novel bacterium with prospective cold active industrial enzymes, isolated from East Rathong glacier in India. J Biotechnol 214:139–140. https://doi.org/10.1016/j.jbiotec.2015.09.025
Kumar R, Singh D, Swarnkar MK et al (2015) Genome assembly of Chryseobacterium polytrichastri ERMR1:04, a psychrotolerant bacterium with cold active proteases, isolated from east rathong glacier in India. Genome Announc 3:2014–2015. https://doi.org/10.1128/genomeA.01305-15
Kumar R, Singh D, Swarnkar MK et al (2016) Complete genome sequence of Arthrobacter alpinus ERGS4:06, a yellow pigmented bacterium tolerant to cold and radiations isolated from Sikkim Himalaya. J Biotechnol 220:86–87. https://doi.org/10.1016/j.jbiotec.2016.01.016
Kumar R, Acharya V, Singh D, Kumar S (2018) Strategies for high-altitude adaptation revealed from high-quality draft genome of non-violacein producing Janthinobacterium lividum ERGS5:01. Stand Genomic Sci 13:1–13. https://doi.org/10.1186/s40793-018-0313-3
Kumar R, Acharya V, Mukhia S et al (2019) Complete genome sequence of Pseudomonas frederiksbergensis ERDD5:01 revealed genetic bases for survivability at high altitude ecosystem and bioprospection potential. Genomics 111:492–499. https://doi.org/10.1016/j.ygeno.2018.03.008
Kumar A, Mukhia S, Kumar N et al (2020) A broad temperature active lipase purified from a psychrotrophic bacterium of Sikkim Himalaya with potential application in detergent formulation. Front Bioeng Biotechnol 8:1–16. https://doi.org/10.3389/fbioe.2020.00642
Sánchez ÓJ, Ospina DA, Montoya S (2017) Compost supplementation with nutrients and microorganisms in composting process. Waste Manag 69:136–153. https://doi.org/10.1016/j.wasman.2017.08.012
Kasana RC, Pandey CB (2018) Exiguobacterium: an overview of a versatile genus with potential in industry and agriculture. Crit Rev Biotechnol 38:141–156. https://doi.org/10.1080/07388551.2017.1312273
Borker SS, Thakur A, Kumar S et al (2021) Correction to: Comparative genomics and physiological investigation supported safety, cold adaptation, efficient hydrolytic and plant growth-promoting potential of psychrotrophic Glutamicibacter arilaitensis LJH19, isolated from night-soil compost (BMC Ge. BMC Genomics 22:1–17. https://doi.org/10.1186/s12864-021-07681-4
Mukhia S, Kumar A, Kumar R (2021) Generation of antioxidant peptides from soy protein isolate through psychrotrophic Chryseobacterium sp. derived alkaline broad temperature active protease. Lwt 143:111152. https://doi.org/10.1016/j.lwt.2021.111152
Wang C, Dong D, Wang H et al (2016) Metagenomic analysis of microbial consortia enriched from compost: new insights into the role of Actinobacteria in lignocellulose decomposition. Biotechnol Biofuels 9:1–17. https://doi.org/10.1186/s13068-016-0440-2
Wang W-K, Liang C-M (2021) Enhancing the compost maturation of swine manure and rice straw by applying bioaugmentation. Sci Rep 11:1–11. https://doi.org/10.1038/s41598-021-85615-6
Zang H, Du X, Wang J et al (2020) Insight into cold-active xylanase production and xylan degradation pathways in psychrotrophic Acinetobacter sp. HC4 from the cold region of China. Cellulose 27:7575–7589. https://doi.org/10.1007/s10570-020-03286-4
Vermelho AB, Couri S (2013) Methods to determine enzymatic activity. Bentham Science Publishers. https://doi.org/10.2174/97816080530011130101
Schleifer KH (2009) Classification of bacteria and archaea: past, present and future. Syst Appl Microbiol 32:533–542. https://doi.org/10.1016/j.syapm.2009.09.002
Kumar R, Nongkhlaw M, Acharya C, Joshi SR (2013) Uranium (U)-tolerant bacterial diversity from U ore deposit of domiasiat in North-East India and its prospective utilisation in bioremediation. Microbes Environ 28:33–41. https://doi.org/10.1264/jsme2.ME12074
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Thite VS (2020) Crude xylanases and pectinases from Bacillus spp. along with commercial cellulase formulate an efficient tailor-made cocktail for sugarcane bagasse saccharification. 286–300. https://doi.org/10.1007/s12155-019-10050-5
Campos LMA, Moura HOMA, Cruz AJG et al (2020) Response surface methodology (RSM) for assessing the effects of pretreatment, feedstock, and enzyme complex association on cellulose hydrolysis. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00756-4
Kumar A, Mukhia S, Kumar R (2021) Production, characterisation, and application of exopolysaccharide extracted from a glacier bacterium Mucilaginibacter sp. ERMR7:07. Process Biochem 113:27–36. https://doi.org/10.1016/j.procbio.2021.12.018
Tandon HLS (2005) Methods of analysis of soils, plants, waters, fertilisers & organic manures. Fertiliser Development and Consultation Organisation
Melo IS, Zucchi TD, Silva RE et al (2014) Isolation and characterization of cellulolytic bacteria from the Stain house Lake, Antarctica. Folia Microbiol (Praha) 59:303–306. https://doi.org/10.1007/s12223-013-0295-x
Zheng G, Lu Z, Li J et al (2020) Screening and performance of L-14, A novel, highly efficient and low temperature-resistant cellulose-degrading strain. Int J Agric Biol Eng 13:247–254. https://doi.org/10.25165/j.ijabe.20201301.5128
Liang Y-L, Zhang Z, Wu M, et al (2014) Isolation, screening, and identification of cellulolytic bacteria from natural reserves in the subtropical region of China and optimization of cellulase production by Paenibacillus terrae ME27–1. Biomed Res Int. https://doi.org/10.1155/2014/512497
Chen L, Wei Y, Shi M et al (2020) Statistical optimization of a cellulase from Aspergillus glaucus CCHA for hydrolyzing corn and rice straw by RSM to enhance yield of reducing sugar. Biotechnol Lett 42:583–595. https://doi.org/10.1007/s10529-020-02804-5
Gil A (2021) Current insights into lignocellulose related waste valorization. Chem Eng J Adv 8:100186. https://doi.org/10.1016/j.ceja.2021.100186
Kumar A, & Gupta N (2018) Potential of lignocellulosic materials for production of ethanol. Biofuels: Greenh Gas Mitig Glob Warm 271–290. https://doi.org/10.1007/978-81-322-3763-1_15
Schedl A, Korntner P, Zweckmair T et al (2016) Detection of Cellulose-Derived Chromophores by Ambient Ionization-MS. Anal Chem 88:1253–1258. https://doi.org/10.1021/acs.analchem.5b03646
Lattuati-Derieux A, Bonnassies-Termes S, Lavédrine B (2006) Characterisation of compounds emitted during natural and artificial ageing of a book. Use of headspace-solid-phase microextraction/gas chromatography/mass spectrometry. J Cult Herit 7:123–133. https://doi.org/10.1016/j.culher.2006.02.004
Li L, Li K, Wang K et al (2014) Efficient production of 2,3-butanediol from corn stover hydrolysate by using a thermophilic Bacillus licheniformis strain. Bioresour Technol 170:256–261. https://doi.org/10.1016/j.biortech.2014.07.101
Yang TH, Rathnasingh C, Lee HJ, Seung D (2014) Identification of acetoin reductases involved in 2,3-butanediol pathway in Klebsiella oxytoca. J Biotechnol 172:59–66. https://doi.org/10.1016/j.jbiotec.2013.12.007
Fouda F (2021) Production of biogas by using different pretreatments of rice straw under aerobic and semi aerobic conditions. Ann Agric Sci Moshtohor 59:349–360. https://doi.org/10.21608/assjm.2021.194823
Li YH, Bai YX, Pan CM et al (2015) Effective conversion of maize straw wastes into bio-hydrogen by two-stage process integrating H2 fermentation and MECs. Environ Sci Pollut Res 22:18394–18403. https://doi.org/10.1007/s11356-015-5016-3
Kaur T, Rana KL, Kour D, et al (2020) Microbe-mediated biofortification for micronutrients: present status and future challenges. Microb Biotechnol and Bioeng 1–17. https://doi.org/10.1016/B978-0-12-820528-0.00002-8
Mikula K, Izydorczyk G, Skrzypczak D, et al (2020) Controlled release micronutrient fertilizers for precision agriculture. Sci Total Environ 712. https://doi.org/10.1016/j.scitotenv.2019.136365
Bradáčová K, Weber NF, Morad-Talab N et al (2016) Micronutrients (Zn/Mn), seaweed extracts, and plant growth-promoting bacteria as cold-stress protectants in maize. Chem Biol Technol Agric 3:1–10. https://doi.org/10.1186/s40538-016-0069-1
Kobayashi T, Nozoye T, Nishizawa NK (2019) Iron transport and its regulation in plants. Free Radic Biol Med 133:11–20. https://doi.org/10.1016/j.freeradbiomed.2018.10.439
Larbi A, Kchaou H, Gaaliche B et al (2020) Supplementary potassium and calcium improves salt tolerance in olive plants. Sci Hortic (Amsterdam) 260:108912
Acknowledgements
We are thankful to the Department of Forest, Govt. of Sikkim, and the Sikkim State Council of Science and Technology for their support in sample collection. The authors duly acknowledge Mrs. Vijaylata Pathania for GC-MS, Dr. Avnesh Kumari for SEM analysis, and Mr. Ramdeen Prasad for AAS analysis. This manuscript represents CSIR-IHBT communication no 5113.
Funding
SK is financially supported by the NMHS project of MoEF&CC (sanction no. GBPNI/NMHS-2018–19/SG/178) to work on the aspect of waste management in the Indian Himalayan regions. AK is supported by the Department of Biotechnology (DBT), Ministry of Science and Technology Government of India (DBT/JRF/BET-17/I/2017/AL/367) through PhD studentship award. The project is financially supported by the DST-TDT project no. DST/TDT/WM/2019/43DST.
Author information
Authors and Affiliations
Contributions
Sareeka Kumari: conceptualization, methodology, investigation, visualization, validation, writing—original draft. Anil Kumar: methodology, investigation, validation, writing—original draft. Rakshak Kumar: conceptualization, resources, supervision, writing—review and editing, finalizing the manuscript, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumari, S., Kumar, A. & Kumar, R. A cold-active cellulase produced from Exiguobacterium sibiricum K1 for the valorization of agro-residual resources. Biomass Conv. Bioref. 13, 14777–14787 (2023). https://doi.org/10.1007/s13399-022-03031-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-03031-w