Abstract
Suaeda glauca (SG), widely applied to restore salinized land, was used to prepare biochar (activated) for the adsorption of bisphenol S (BPS) from water. SG had a high cellulose content and uniform salt ion distribution. The migration pathway of salt ions in halophytes was analyzed via energy dispersive spectrometer (EDS), inductively coupled plasma atomic emission spectrometry (ICP-AES), and X-ray diffraction (XRD). Phosphoric acid impregnation could lead to the leaching of 80.52% of salt ions from SG. The products had high specific surface area (1339 m2/g) and adsorption capacity for BPS (437 mg/g). Response surface methodology indicated that pH and temperature could significantly influence the adsorption capacity. Molecular dynamics and quantum chemistry were used to describe the adsorption process and mechanism. By comparing the pore size distribution and adsorption capacity of different carbons with the simulation results of molecular dynamics, it can be discovered that the effect of micropore size on pollutant adsorption is verifiable through graphite layer modeling. Meanwhile, the presence of salt ions had no significant effect on BPS adsorption capacity. The absorption mechanism might be ascribed to a large specific surface area and π–π bond interactions.
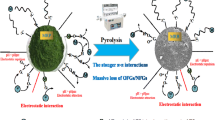
Graphical abstract










Similar content being viewed by others
Data availability
Supplementary data to this article can be found online.
References
Zdarta J, Antecka K, Frankowski R et al (2018) The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci Total Environ 615:784–795. https://doi.org/10.1016/j.scitotenv.2017.09.213
Fang Z, Gao Y, Wu X et al (2020) A critical review on remediation of bisphenol S (BPS) contaminated water: efficacy and mechanisms. Crit Rev Environ Sci Technol 50:476–522. https://doi.org/10.1080/10643389.2019.1629802
Sun P, Liu X, Zhang M et al (2021) Sorption and leaching behaviors between aged MPs and BPA in water: the role of BPA binding modes within plastic matrix. Water Res 195:116956. https://doi.org/10.1016/j.watres.2021.116956
Zhao C, Xie P, Yong T et al (2018) MALDI-MS imaging reveals asymmetric spatial distribution of lipid metabolites from bisphenol S-induced nephrotoxicity. Anal Chem 90:3196–3204. https://doi.org/10.1021/acs.analchem.7b04540
Ahsan N, Ullah H, Ullah W, Jahan S (2018) Comparative effects of bisphenol S and bisphenol A on the development of female reproductive system in rats; a neonatal exposure study. Chemosphere 197:336–343. https://doi.org/10.1016/j.chemosphere.2017.12.118
Ullah H, Ambreen A, Ahsan N, Jahan S (2017) Bisphenol S induces oxidative stress and DNA damage in rat spermatozoa in vitro and disrupts daily sperm production in vivo. Toxicol Environ Chem 99:953–965. https://doi.org/10.1080/02772248.2016.1269333
Liao C, Kannan K (2014) A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Addit Contam - Part A Chem Anal Control Expo Risk Assess 31:319–329. https://doi.org/10.1080/19440049.2013.868611
Liao C, Liu F, Alomirah H et al (2012) Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol 46:6860–6866. https://doi.org/10.1021/es301334j
Jin H, Zhu L (2016) Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake, China. Water Res 103:343–351. https://doi.org/10.1016/j.watres.2016.07.059
Yamazaki E, Yamashita N, Taniyasu S et al (2015) Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol Environ Saf 122:565–572. https://doi.org/10.1016/j.ecoenv.2015.09.029
Nejumal KK, Dineep D, Mohan M et al (2018) Presence of bisphenol S and surfactants in the sediments of Kongsfjorden: a negative impact of human activities in Arctic? Environ Monit Assess. https://doi.org/10.1007/s10661-017-6383-7
Shao P, Ren Z, Tian J et al (2017) Silica hydrogel-mediated dissolution-recrystallization strategy for synthesis of ultrathin Α-Fe2O3 nanosheets with highly exposed (1 1 0) facets: a superior photocatalyst for degradation of bisphenol S. Chem Eng J 323:64–73. https://doi.org/10.1016/j.cej.2017.04.069
Yang T, Wang L, Liu Y et al (2019) Comparative study on ferrate oxidation of BPS and BPAF: kinetics, reaction mechanism, and the improvement on their biodegradability. Water Res 148:115–125. https://doi.org/10.1016/j.watres.2018.10.018
Choi YJ, Lee LS (2017) Aerobic soil biodegradation of bisphenol (BPA) alternatives bisphenol S and bisphenol AF compared to BPA. Environ Sci Technol 51:13698–13704. https://doi.org/10.1021/acs.est.7b03889
Ren Y, Wang S, Zhang J et al (2021) Enhancing the performance of Fenton-like oxidation by a dual-layer membrane: a sequential interception-oxidation process. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.123766
Reddy PVL, Kim KH, Kavitha B et al (2018) Photocatalytic degradation of bisphenol A in aqueous media: a review. J Environ Manage 213:189–205. https://doi.org/10.1016/j.jenvman.2018.02.059
Kim S, Chu KH, Al-Hamadani YAJ et al (2018) Removal of contaminants of emerging concern by membranes in water and wastewater: a review. Chem Eng J 335:896–914. https://doi.org/10.1016/j.cej.2017.11.044
Wang B, Gao B, Fang J (2017) Recent advances in engineered biochar productions and applications. Crit Rev Environ Sci Technol 47:2158–2207. https://doi.org/10.1080/10643389.2017.1418580
Feng Q, Wang B, Chen M et al (2021) Invasive plants as potential sustainable feedstocks for biochar production and multiple applications: a review. Resour Conserv Recycl 164:105204. https://doi.org/10.1016/j.resconrec.2020.105204
Cheng N, Wang B, Wu P et al (2021) Adsorption of emerging contaminants from water and wastewater by modified biochar: a review. Environ Pollut 273:116448. https://doi.org/10.1016/j.envpol.2021.116448
Wang T, Xue L, Zheng L et al (2021) Biomass-derived N/S dual-doped hierarchically porous carbon material as effective adsorbent for the removal of bisphenol F and bisphenol S. J Hazard Mater 416:126126. https://doi.org/10.1016/j.jhazmat.2021.126126
Ahmadpour A, Do DD (1996) The preparation of active carbons from coal by chemical and physical activation. Carbon N Y 34:471–479. https://doi.org/10.1016/0008-6223(95)00204-9
Chenfei SHI, Yumeng LI, Haiyao F et al (2018) Removal of p -nitrophenol using persulfate activated by biochars prepared from different biomass materials. Chem Res Chin Univ 34:39–43. https://doi.org/10.1007/s40242-017-7245-0
Prahas D, Kartika Y, Indraswati N, Ismadji S (2008) Activated carbon from jackfruit peel waste by H3PO4 chemical activation: pore structure and surface chemistry characterization. Chem Eng J 140:32–42. https://doi.org/10.1016/j.cej.2007.08.032
Cheng N, Wang B, Feng Q et al (2021) Co-adsorption performance and mechanism of nitrogen and phosphorus onto eupatorium adenophorum biochar in water. Bioresour Technol 340:125696. https://doi.org/10.1016/j.biortech.2021.125696
Kefu Z, Hai F, Ungar IA (2002) Survey of halophyte species in China. Plant Sci 163:491–498. https://doi.org/10.1016/S0168-9452(02)00160-7
Yang C, Shi D, Wang D (2008) Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Regul 56:179–190. https://doi.org/10.1007/s10725-008-9299-y
Flowers TJ (1972) Salt Tolerance in Suaeda maritima (L.) Dum: the effect of sodium chloride on growth, respiration, and soluble enzymes in a comparative study with pisum sativum SATIVUM L. J Exp Bot 23:310–321. https://doi.org/10.1093/jxb/23.2.310
Sun HX, Zhou DW, Zhao CS et al (2012) Evaluation of yield and chemical composition of a halophyte (Suaeda glauca) and its feeding value for lambs. Grass Forage Sci 67:153–161. https://doi.org/10.1111/j.1365-2494.2011.00831.x
Siatecka A, Różyło K, Ok YS, Oleszczuk P (2021) Biochars ages differently depending on the feedstock used for their production: willow- versus sewage sludge-derived biochars. Sci Total Environ 789:147458. https://doi.org/10.1016/j.scitotenv.2021.147458
Chen Y, Liu J, Zeng Q et al (2021) Preparation of Eucommia ulmoides lignin-based high-performance biochar containing sulfonic group: synergistic pyrolysis mechanism and tetracycline hydrochloride adsorption. Bioresour Technol 329:124856. https://doi.org/10.1016/j.biortech.2021.124856
McLintock IS (1967) The elovich equation in chemisorption kinetics. Nature 216:1204–1205. https://doi.org/10.1038/2161204a0
Rahmani-Sani A, Singh P, Raizada P et al (2020) Use of chicken feather and eggshell to synthesize a novel magnetized activated carbon for sorption of heavy metal ions. Bioresour Technol 297:122452. https://doi.org/10.1016/j.biortech.2019.122452
Gorgievski M, Božić D, Stanković V et al (2013) Kinetics, equilibrium and mechanism of Cu2+, Ni2+ and Zn2+ ions biosorption using wheat straw. Ecol Eng 58:113–122. https://doi.org/10.1016/j.ecoleng.2013.06.025
Awan S, Ippolito JA, Ullman JL et al (2021) Biochars reduce irrigation water sodium adsorption ratio. Biochar 3:77–87. https://doi.org/10.1007/s42773-020-00073-z
Liu H, Zhang J, Bao N et al (2012) Textural properties and surface chemistry of lotus stalk-derived activated carbons prepared using different phosphorus oxyacids: adsorption of trimethoprim. J Hazard Mater 235–236:367–375. https://doi.org/10.1016/j.jhazmat.2012.08.015
Yang H, Chen P, Chen W et al (2022) Insight into the formation mechanism of N, P co-doped mesoporous biochar from H3PO4 activation and NH3 modification of biomass. Fuel Process Technol 230:107215. https://doi.org/10.1016/j.fuproc.2022.107215
Wang S, Gao B, Zimmerman AR et al (2015) Physicochemical and sorptive properties of biochars derived from woody and herbaceous biomass. Chemosphere 134:257–262. https://doi.org/10.1016/j.chemosphere.2015.04.062
Pei Z, Li L, Sun L et al (2013) Adsorption characteristics of 1,2,4-trichlorobenzene, 2,4,6- trichlorophenol, 2-naphthol and naphthalene on graphene and graphene oxide. Carbon N Y 51:156–163. https://doi.org/10.1016/j.carbon.2012.08.024
Kayiranga A, Luo Z, Ndayishimiye JC et al (2021) Insights into thallium adsorption onto the soil, bamboo-derived biochar, and biochar amended soil in pomelo orchard. Biochar 3:315–328. https://doi.org/10.1007/s42773-021-00095-1
Suárez-García F, Martínez-Alonso A, Tascón JMD (2004) Activated carbon fibers from Nomex by chemical activation with phosphoric acid. Carbon N Y 42:1419–1426. https://doi.org/10.1016/j.carbon.2003.11.011
Montané D, Torné-Fernández V, Fierro V (2005) Activated carbons from lignin: kinetic modeling of the pyrolysis of Kraft lignin activated with phosphoric acid. Chem Eng J 106:1–12. https://doi.org/10.1016/j.cej.2004.11.001
Suhas CPJM, Ribeiro Carrott MML (2007) Lignin - from natural adsorbent to activated carbon: a review. Bioresour Technol 98:2301–2312. https://doi.org/10.1016/j.biortech.2006.08.008
Babeł K, Jurewicz K (2008) KOH activated lignin based nanostructured carbon exhibiting high hydrogen electrosorption. Carbon N Y 46:1948–1956. https://doi.org/10.1016/j.carbon.2008.08.005
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Prasannamedha G, Kumar PS, Mehala R et al (2021) Enhanced adsorptive removal of sulfamethoxazole from water using biochar derived from hydrothermal carbonization of sugarcane bagasse. J Hazard Mater 407:124825. https://doi.org/10.1016/j.jhazmat.2020.124825
Vijayaraghavan K, Padmesh TVN, Palanivelu K, Velan M (2006) Biosorption of nickel(II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models. J Hazard Mater 133:304–308. https://doi.org/10.1016/j.jhazmat.2005.10.016
Saadi R, Saadi Z, Fazaeli R, Fard NE (2015) Monolayer and multilayer adsorption isotherm models for sorption from aqueous media. Korean J Chem Eng 32:787–799. https://doi.org/10.1007/s11814-015-0053-7
Cheng Q, Huang Q, Khan S et al (2016) Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecol Eng 87:240–245. https://doi.org/10.1016/j.ecoleng.2015.11.045
Xiong Z, Huanhuan Z, Jing W et al (2021) Physicochemical and adsorption properties of biochar from biomass-based pyrolytic polygeneration: effects of biomass species and temperature. Biochar 3:657–670. https://doi.org/10.1007/s42773-021-00102-5
Wu LH, Zhang XM, Wang F et al (2018) Occurrence of bisphenol S in the environment and implications for human exposure: a short review. Sci Total Environ 615:87–98. https://doi.org/10.1016/j.scitotenv.2017.09.194
Li L, Xu D, Pei Z (2016) Kinetics and thermodynamics studies for bisphenol S adsorption on reduced graphene oxide. RSC Adv 6:60145–60151. https://doi.org/10.1039/c6ra10607b
Huang Q, Song S, Chen Z et al (2019) Biochar-based materials and their applications in removal of organic contaminants from wastewater: state-of-the-art review. Biochar 1:45–73. https://doi.org/10.1007/s42773-019-00006-5
Wang Z, Lv Q, Chen S et al (2018) Molecular dynamics simulations on heterogeneity and percolation of epoxy nanofilm during glass transition process. Mater Chem Phys 213:239–248. https://doi.org/10.1016/j.matchemphys.2018.04.040
Jin Z, Wang X, Sun Y et al (2015) Adsorption of 4- n -nonylphenol and bisphenol-A on magnetic reduced graphene oxides: a combined experimental and theoretical studies. Environ Sci Technol 49:9168–9175. https://doi.org/10.1021/acs.est.5b02022
Acknowledgements
We want to acknowledge anonymous reviewers for their valuable comments.
Funding
This work was supported by the Science and Technology Major Projects of Shandong Province (2020CXGC011406), Natural Science Foundation of Shandong Province, China (ZR2019QEE034), National Natural Science Foundation of China (51908343), Youth Innovation Technology Project of Higher School in Shandong Province (2019KJD003) and the Introduction and Cultivation Plan for Young Innovative Talents of Colleges and Universities by the Education Department of Shandong Province.
Author information
Authors and Affiliations
Contributions
Conceptualization: FS and JX. Methodology: FS, JX, YJ, CZ, and XZ. Formal analysis and investigation: FS. Writing — original draft preparation: FS. Writing — review and editing: FS and JX. Funding acquisition: JX, CZ, and RM. Supervision: JX, CZ, CL, YF, JZ, and RM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shao, F., Xu, J., Jing, Y. et al. Pyrolytic utilization of a typical halophyte: Suaeda glauca—the excellent adsorbent raw material for bisphenol S removal. Biomass Conv. Bioref. 14, 8041–8055 (2024). https://doi.org/10.1007/s13399-022-02859-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02859-6