Abstract
Anaerobic digestion (AD) is a well-known biological conversion process to obtain a gaseous biofuel from organic matter: in fact, upgrading biogas to biomethane is a mean to substitute conventional natural gas. It is also known that biochar can improve the biogas production in AD processes. In this work, different biochars have been produced from various feedstocks at different process conditions. Biochars obtained from the carbonization of wheat straw (WS) and poplar (P) were produced in a Thermo Gravimetric Analyser at lab scale, at a temperature of 400 °C and 2 h of retention time at the maximum temperature, with a heating rate of 20 °C min−1. Another biochar from poplar (Pc) was also produced in a pilot plant (CarbOn, RE-CORD) working in oxidative pyrolysis conditions, at a temperature range between 500 and 600 °C. Biochars were oxidized with Oxone® using two different methods (ball-milling and simple aqueous solution mixing) to increase the amount of functional groups on their surface. Oxidized biochars (Ws_Ox and P_Ox) were characterized by FTIR, BET, and CEC, and their impact on biogas production was investigated through a lab scale biochemical methane potential (BMP) test using maize silage as substrate. 0.33 g of biochar was used for each treatment. BMP test shows that all batches containing biochar as additive produced more biogas than control (C). WS_Ox and P_Ox produced respectively a + 7.7% and + 11.3% of biogas than C, obtaining the higher productivities with respect to not oxidized biochars. The addition of P and Pc biochars were similar performances in AD, thus highlighting that no significant differences are due to different biochar production scales and process parameters from the same feedstock. This study highlights how in addition to the various examined parameters (nature of the feedstock, pyrolysis parameters, size of biochar and its concentration in AD), also the presence of specific functional groups on the biochar surface influences the AD performance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In order to mitigate the impacts of greenhouse gases (GHG) on planet’s climate, it is necessary to make an energy transition, trying to progressively substitute fossil fuels with renewable energy resources. A sustainable way to produce biofuels from organic matter has been identified in the anaerobic digestion (AD) process. AD involves the decomposition and degradation of organic material under anaerobic conditions by a different microbial population, finally resulting in the production of a gas mixture, named biogas. The AD performance needs to be improved to make the process more economically viable [1]. Optimizations of the process control variables were reported to minimize energy consumption and increase biogas production [2].
The microbiological activity and, consequently, the production of biogas are strictly related to the process parameters. To improve the efficiency of substrate degradation, pretreatments are sometimes required to overcome the recalcitrance of raw materials and improve AD performance [3]. The AD process is carried out by a consortium of microorganisms that breaks down complex organic material under anoxic conditions in multiple steps. The microorganisms that drive AD are classified as the acidogens, acetogens, and methanogens [4]. The acidogens degrade soluble organic molecules and produce volatile fatty acids (VFA), which are used as a substrate by acetogens to carry out their metabolic pathways. As a result of their metabolism, acetogens discard acetic acid, carbon dioxide, and hydrogen. Methanogen bacteria use residual molecules released from the above-mentioned free-living organisms as a substrate to produce mainly CH4 and CO2 [5]. Conversions of complex organic compounds into CH4 and CO2 are possible thanks to the cooperation of the different groups of microorganisms. Syntrophy is a form of symbiosis of two metabolically different groups of bacteria, which allows the degradation of various substrates [6]. Commonly, microorganisms use a specific structure, called pilus, as electron transfer conduit to enhance the efficiency of syntrophic metabolism. The presence of conductive materials into the digester efficiently helps to stimulate the direct interspecies electron transfer (DIET) between microbial population [7]. Some conductive materials improve the DIET and consequently the biogas production. Materials with a higher electrical conductivity are suited for this purpose, like biochar, nickel foam, and metals particles [8,9,10]. Biochar is a carbon-based material obtained through thermal decomposition of biomass by means of a pyrolytic process. Biochar physical characteristics like porosity (specific surface area and pore size distribution), bulk density, and surface chemistry like cation exchange capacity (CEC), pH, and electrical conductivity (EC) mainly depend not only on pyrolysis process parameters [11] but also on biomass characteristics [12]. Thanks to its properties, biochar can be widely used in various environmental applications such as soil remediation, water and gas filtering, human food-supplement, animal feed, pharmaceutic product, building material, and as additive in anaerobic digestion [13,14,15]. In the latter case biochar could improve biogas production, as conductive material but also an adsorbent, and may have a positive impact on process stability and quality of the digestate produced.
The stability of the process is reached by four different mechanisms like adsorption of inhibitors, buffering capacity of the environmental pH, immobilization of bacterial cells within pores [16, 17], and DIET. All these mechanisms are strictly linked to the chemical and physical properties of biochar. For biochar, an important parameter is represented by the ion exchange capacity (IEC), which represents the capability of solid materials to exchange ions with organic or inorganic matter. In particular, the high presence of surface functional groups, as carboxyl and phenolic groups, allows to increase the CEC values and hence the capability to adsorb cations [18]. As a consequence, thanks to the improvement of microbial community life conditions in the anaerobic digester, it increases the biogas production and the stability of the AD process. Furthermore, the higher microporosity of biochar could trap toxic compounds and metal ions that inhibit the microorganism proliferations. The mesoporosity and macroporosity of biochars often are inhabited by free-living organisms and ameliorate the efficiency of the AD process.
In recent year, many studies summarized the positive effects produced by the introduction of biochar into the AD process [5, 19,20,21,22,23]. For example, Zhang L. et al. [24] used a biochar, produced by gasification of waste wood pellets from the sawmill at 700–800 °C in a small-scale autothermal gasifier, for improving the thermophilic semicontinuous anaerobic digestion (AD) of food waste to produce methane; the optimal dosage range of biochar resulted between 7.5 and 15 g L−1 with particle size less than 1 mm.
To date, whether a biochar with high CEC could be preferable for improving biogas productivity in an AD system has yet to be demonstrated. M. O. Fagbohungbe et al. in their study showed that high CEC value could achieve the highest removal efficiency of dangerous molecules [25]. Moreover, there is little information on which type of raw materials should be fed to a pyrolysis reactor and the optimal process parameters to produce a tailored biochar as additive for AD. In our work, two different raw materials were selected to obtain biochar: wheat straw and poplar wood chips, deriving respectively from agricultural waste and agroforestry activities. The biochars were produced in a Thermo Gravimetric Analyser at lab scale and in a pilot plant (CarbOn, RE-CORD) operating in oxidative pyrolysis conditions. To understand if the CEC properties could influence the AD performances, biochars have been oxidized to increase the surface functional groups content. The performance in biogas production of the biochars were evaluated with biochemical methane potential (BMP) test using the method of Angelidaki I. et al. [26].
2 Materials and methods
2.1 Chemicals
All the reagents and solvents used in this study are purchased from Sigma-Aldrich and Carlo Erba. They were used without any further purification. Gases were purchased from Rivoira (Italy). Helium had a purity of 99.9995%, while all the other gases’ purity was 99,9999%.
2.2 Inoculum, feedstock, and substrate
The inoculum used for BMP test derived from an anaerobic digestion plant of 1 MWel, located in Tuscany (Italy). The digester was mainly fed with maize silage. The working temperature of the inoculum in this study was between 46 and 50 °C, replicating the conditions of the digester where it was taken.
Feedstocks used for biochars production were wheat straw (f_WS), a well-known agricultural residue, and poplar chips (f_P) collected from Mugello area in Tuscany (Italy), derived from agroforestry activities. f_WS was pelletized before milling. All the feedstocks were dried and milled to reach 0.25 mm of particle size in a Retsch SM-300 knife mill. Table 1S in Supplementary Information shows the characterization of the two feedstocks by means of proximate and ultimate analyses, content in metals and inorganics.
The substrate used in BMP test was maize silage, one of feedstock of the anaerobic digestion plant. The substrate was stored at − 20 °C, defrosted and dried before BMP tests.
2.3 Biochar preparation
Biochars from wheat straw (herbaceous feedstock) and from poplar wood chips (woody feedstock) were produced in a TGA analyzer (Leco TGA 701) at 400 °C, with a retention time (RT) of 2 h and a heating rate (HR) of 20 °C min−1, under a nitrogen flow (10 L min−1). About 37 g per biochar sample was obtained and labeled respectively as WS and P.
The biochar labeled Pc derived from poplar chips and it was produced in the CarbOn pilot plant, an autothermal carbonization unit developed by RE-CORD [27], in a temperature range between 500 and 600 °C.
The obtained biochar was milled at 0.5 mm.
Thus, two biochars were produced using the same feedstock (P and Pc) but at different pyrolysis processes conditions, technologies, reactor size, and process parameters, so to investigate the effects of different biochar chemical-physical characteristics and their influence in the anaerobic digestion process.
All samples were characterized in terms of elemental analysis, surface area, and functional groups and tested to determine the ion retention and release capability via CEC. Two different oxidation methods have been used to produce oxidized biochar (using WS and P): ball-mill oxidation and oxidation in aqueous solution.
In the ball-mill oxidation, a Powteq GT300 ball-mill was used with Oxone®. 0.1 g of biochar has been introduced in a steel jar with two steel balls (7 mm diameter and 4 g in weight), then 1 g of Oxone® was added. The milling lasted for 12 h at 300 rpm. The time was divided into cycles of 40 min of work and 10 min of rest [28]. The experiments were repeated later testing different Oxone®:biochar ratios: 10:1, 5:1, 1:1. For all experiments, the modified biochar was washed with water and its pH adjusted at 5 using a Na2CO3 solution at 1%wt concentration, then all samples were dried overnight. The samples obtained with the ball-mill oxidation method, using WS and P biochars, were named respectively BM_WS_Ox and BM_P_Ox.
The protocol used for testing oxidation in aqueous solution was reported in the paper of Madduri S. et al. [29]: in 500 mL of water were added 6 g of biochar, 13 g of Oxone®, and 1.3 g of NaCl as catalyst. The solution was then stirred for 24 h at 22 °C. Once this step was concluded, the obtained biochars were washed with H2O and their pH adjusted at 5 through Na2CO3 solution at 1%wt concentration. After a further washing step, the samples were dried in stove at 60 °C overnight. The samples obtained with the aqueous solution oxidation method, using WS and P biochars, were named respectively WS_Ox and P_Ox.
2.4 Ultimate and proximate analyses
The ultimate analysis of feedstocks and biochars was determined according to the UNI EN ISO 16948 and ASTM D4239 standards, using a LECO TruSpec CHN. The proximate composition of feedstocks and biochars was determined according to UNI EN ISO 18122, UNI EN ISO 18123, UNI EN ISO 18134–2 standards, using a LECO TGA 701.
2.5 Surface area
The specific surface area of biochars was determined by N2 adsorption isotherms with Bruneuer-Emmett-Teller method (BET) in a Quantachrome NOVA 2200E instrument. Experiments were performed on 60 mg of samples preliminarily dried at 200 °C for 48 h. All measurements were performed after degassing (200 °C for 24 h).
2.6 Cation exchange capacity measurement
The analysis of CEC has been conducted according to the method provided by the Italian decree for soil analysis DM 13/9/99, method XIII.2. Two grams of biochar have been transferred in a falcon tube; the mass of the sample plus the falcon tube was determined and used in Eq. (1) as term A. A washing step was performed with 30 mL aliquot of 0.1 M BaCl2 with a stirring period of 1 h. Then, the solution has been centrifuged at 3000 rpm for 10 min and subsequently the supernatant was removed. This washing step was repeated two more times.
The sample obtained was washed with 30 mL of water, then centrifuged and the supernatant discarded. The falcon tube was weighted, and the mass value was used in Eq. (1) as B term. After that, the sample was washed with 30 mL of a 0.02 M MgSO4 solution. The dispersion was then stirred for 2 h, then centrifuged. 10 mL of supernatant was collected in a conical flask, in which 100 mL of H2O, 10 mL of NH4Cl, and the Eriochrome Black T indicator were added. The ethylenediaminetetraacetic acid (EDTA) volume used for titration was recorded and used in Eq. (1) as the term M.
CEC was then expressed as per the following equation:
In which:
CEC—cation exchange capacity.
Va—volume of EDTA solution used for titration of sample in mL.
Vb—volume of EDTA solution used for titration of white in mL.
A—sample + falcon tube mass.
B—sample + falcon tube after saturation with BaCl2 and washing with H2O.
M—molar concentration of EDTA solution, in this case 0.0025 M.
2.7 Infrared spectroscopy FT-IR
Infrared spectroscopy was performed in a SHIMADZU IRTracer-100 spectrophotometer. Data were collected at room temperature in attenuated total reflectance (ATR) mode. The optical resolution was 4 cm−1 and the spectral range investigated was from 600 to 4000 cm−1. Each spectrum was averaged over 45 scans.
2.8 Determination of pH
pH was calculated following the method ISO 10390: 5 mL of sample and 25 mL of a water solution 0.1 M of CaCl2 were added in a falcon tube. The solution was shaken for 60 min, using a mechanical shaker. The pH was measured at room temperature with a Metrohm pHlab phmeter.
2.9 Electrical conductivity (EC)
Electrical conductivity was analyzed according to DIN ISO 11265: 5 g of biochar was weighted and transferred in a falcon tube, then 50 mL of water was added (1:10 ratio). The dispersion obtained was shaken for 30 min and then the solids were filtered through filter paper. The EC value was then measured with an Eutech COND 6 + instrument.
2.10 Inductively coupled plasma mass spectrometry (ICP-MS)
Microelement and macroelement contents on inoculum, substrate, feedstocks, and biochars were analyzed after a mineralization step using an Agilent microwave plasma atomic emission spectrometers (A200 MP-AES), previously calibrated with a multielemental standard solution. 0.05 g of biochar and 0.5 g of inoculum and substrate were weighted. Three milliliters of H2O2 and 8 mL of HNO3 were then added to the sample. Samples were then mineralized in a Milestone Microwave Digestor System. HNO3 at 1% concentration was added to the collected mineralized solution to reach 25 mL in volume.
2.11 Biochemical methane potential (BMP)
The method reported by Angelidaki I. et al. [26] was used for BMP test. In a 100 mL vessel, were added a fixed quantity of inoculum (33 mL), then water and an adequate mass of substrate in order to reach a food to microrganism ratio (F/M) of 0.5, majorly used in these studies [30]. The F/M ratio was calculated considering the volatile solids VS content (100-ashd.b.) of the inoculum (M) and the substrate (F). Control vessel contained 34 g of inoculum (total solid content of 2.42 g dry basis) and 0.83 g dry basis of maize silage, biochar addition consisted in 0.33 g of vegetable carbon as additive.
The vessels were closed by gas tight caps and equipped with 100 mL syringes and maintained at 48–50 °C and the BMP test ended after 37 days.
The produced biogas was daily collected through observation of volume reported in the syringes. In the trial, 6 different treatments were tested in triplicate, where (C) is the control, made by the inoculum, water, and corn silage as substrate, and the other five treatments represent the control plus the addition of different biochars: wheat straw biochar (WS), poplar biochar (P), poplar biochar produced in CarbON (Pc), wheat straw oxidized biochar (WS_Ox), and poplar oxidized biochar (P_Ox).
According to previous studies conducted by our team [31], the amount of biochar used for each treatment was 5 g L−1 d.b.
The theoretical biomethane potential was calculated through the relations reported by Buswell, A. M., & Mueller, H. F. [32].
In which:
Biogas is composed of different molecules: CH4, CO2, NH3, and H2S; from the equations described above, it was possible to quantify the theoretical biomethane production (TBMP) adding the individual contributions calculated for each compound present in the biogas mixture. The individual TBMP contributions were calculated through the formula reported below:
where, C%, H%, N%, O%, and S% are collected from CHNS(O) analysis.
Finally, the theoretical biomethane potential was calculated adding each contribution [33].
3 Results and discussion
3.1 Biochar characteristics
In Table 1, the summary of the main characteristics of the produced biochars is reported.
The ICP analysis of biochar WS, P, and Pc is reported in Table 2S of the Supplementary Information. As expected from literature [34], ash content for WS was significantly higher than in P and Pc. In particular, Al, Ca, K, Mg, Mn, and Na are more abundant in WS than in P. The EC reflected the trend showed for % dry weight of the ash content; in fact, it was higher in WS than in poplar biochars (P and Pc). After pyrolysis, fixed C increases in all biochars, but the C/N ratio was higher in P and Pc than in WS. All biochars show a basic pH value and in particular WS resulted with the highest pH value. BET values were variable and the differences seem mainly related to the technology used for their production. WS resulted in a specific surface area lower than P, probably due to the high ash content, characteristic of the feedstock. Pc resulted with the highest specific surface area. Particle sizes used for BMP test are the same for WS and P biochars, the ones produced in TGA (< 0.25 mm), and higher for Pc, the one produced in CarbOn pilot plant (< 0.5 mm) [31].
3.2 Biochar oxidation
FTIR analysis was used to choose the best oxidation method to produce the oxidized biochar. As already described above, the biochars were oxidized with two different methods: by ball-mill (with different ratio biochar:Oxone®) and by aqueous solution (using the same oxidizing agent).
Figure 1 shows the IR spectra of P (a) and WS (b) biochars oxidized with the ball mill method.
The spectra (Fig. 1) showed an adsorption band at 1700 cm−1, which correspond to a carboxylic group, and an absorption band at 1590 cm−1, related to C = C stretching vibration of conjugated systems. C = O groups are particularly suited to drive the CEC value thanks to their capability to create a negative charge on biochar surface. As consequence of the Oxone® concentration increase, the peaks relative to C = O become more evident both in P and WS biochar, but the intensity of C = C peaks decreased. Therefore, a better oxidation with the ball mill method is observed for higher biochar:Oxone® ratios (1:10), but a good degree of oxidation is however obtained with lower Oxone® concentrations (ratios 1: 5 and 1: 2.5).
Figure 2 shows the IR spectra of P (a) and WS (b) biochars oxidized with the aqueous phase method.
In this case, spectra in Fig. 2 show the peak at ~ 1700 cm−1 related to the stretching of C = O and the peak at 1597 cm−1 related to C = C stretching as resulted with the ball mill method. Although it is possible to obtain excellent results with the ball mill method, it requires large amount of reagent and long reaction time. Furthermore, the volume of the vessel required and the heat produced during the grinding phase are the main bottleneck related to a possible industrial-scale implementation of this method. Vice versa, the reaction in aqueous solution allowed to obtain excellent results with a reasonable biochar:Oxone® ratio.
The ICP analysis of oxidized biochars is reported in Table 3S of the Supplementary Information.
Table 2 shows the BET and CEC values obtained for WS_Ox and P_Ox. Compared to the corresponding not oxidized biochars, WS_Ox and P_Ox did not result with significant variations in their specific surface area (BET value). On the other hand, they showed a significant increase in CEC value with respect to unoxidized biochars samples: + 23.1% and + 40.1% respectively.
3.3 BMP test
Figure 3 shows the trend in biogas production over the 37 days in which the test was conducted.
In all treatments where biochar was added, more biogas than the control was produced.
Regarding the untreated biochars, P produced more than Pc and WS, respectively 574 ± 25.8 mL, 564 ± 54.4 mL and 554 ± 145.9 mL of biogas. The maximum difference between untreated biochar and control in terms of productivity is recorded on the 15th day from the start of the test, when a yield greater than + 13.4% with WS, + 20.2% with P, and + 16% with Pc was obtained. In conclusion, the obtained results confirm the positive effect in biogas production due to the presence of biochar in AD, as reported in many papers and described above [16, 17, 24].
As already described above, P and Pc are biochars produced with different pyrolysis temperatures and different technologies and scales, but the differences in biogas yield are not very significant. Furthermore, in the analysis on the effects of the process, it must be considered that, for the two samples P and Pc, the specific surface area values (BET) resulted in a different order of magnitude. BET analysis mainly investigates the porosity in a pore size range from microporosity to mesoporosity (< 50 nm), not appreciating the contribution of macroporosity. To understand if this contribution has specific effects on the process (e.g., favoring the inclusion of microorganisms in the surfaces of the biochar particles), it is suggested to investigate it with a mercury porosimetry combined with a scanning electric microscope (SEM).
P was produced at a lower temperature than Pc, using the same feedstock: it can therefore be observed that biochar produced at a lower temperature had similar effects of a biochar produced at a higher temperature. Reducing the process temperature from 500 to 400 °C can lead to economic benefits for the operation of a slow-pyrolysis plant. The main difference among the two biochars (P and Pc) so produced consists in the ash content: WS ash % w/w is higher than P; that difference probably drove the high standard deviation of WS batch triplicates.
In Fig. 3, it is also possible to observe different effects due to the feedstock used for biochar production; in fact, WS biochar sample has undergone the same production process of P but resulted in a lower average biogas production with a high standard deviation (as shown in Fig. 4).
Results for the oxidized biochar samples (WS_Ox and P_Ox) show that their cumulative biogas production’s trend followed the shape of the control (C) but with respect to the latter, the biogas yield resulted higher for both: at the 37th day it was 584 ± 35.8 mL for WS_Ox and 603 ± 53.43 mL for P_Ox (Fig. 3). P_Ox showed a better average result value than WS_Ox, confirming for the oxidized biochars the same results obtained for the not oxidized samples: the poplar feedstock performed better than the wheat straw one.
Finally, it is possible to notice that in the first two days there is a boost in biogas yield in both WS_Ox and P_Ox with respect to the not treated biochars, but later, their production alignes with the other treatments. Biogas achieved the highest yield on the 21st day: WS_Ox and P_Ox produced respectively + 14.9% and + 20.9% more than the control (C).
Making a comparison between oxidized biochar and untreated biochar, it is possible to observe how the treated biochars (WS_Ox and P_Ox) resulted in a higher biogas yield than the untreated ones (WS, P and Pc). In fact, the biogas yield at the end of the BMP test (on 37th day) was higher for WS_Ox compared to WS (+ 5.5%) and for P_Ox compared to P (+ 5.4%) and to Pc (+ 7.2%); so the results show that biochar oxidation promotes the AD process performance. Furthermore, WS_Ox resulted with a stronger reduction of the standard deviation value than the not oxidized biochar WS; the difference between the two biochar samples is mainly the higher CEC value obtained with the proposed oxidation process for WS_Ox (Table 2).
3.4 BMP test results with respect to the theoretical production
Applying the Buswell’s formula for corn silage, the theoretical biogas potential resulted of 662 ± 56.82 mL (as reported in Fig. 4). Data of cumulative biogas production showed that P_Ox and WS_Ox are the treatments that produced the most quantity of biogas, respectively with a 603 ± 53.4 mL and 584 ± 35.8 mL.
Treatments with not oxidized biochar WS, P, and Pc attained cumulative biogas production of 554 ± 145.9 mL, 574 ± 25.8 mL, and 564 ± 54.4 mL, respectively.
Control (C) is recorded as the lowest value of biogas produced with 542 ± 13.9 mL.
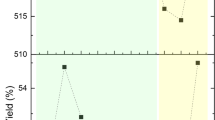
Table 3 compares biogas yields as % of the different biochars versus Control at different days of the BMP test (15th, 21st, 25th, 30th, and 37th). The data are also shown in graphical form in Fig. 5.
Commonly, hydraulic retention time HRT refers to the mean time a particle is held into the digester and its optimal value may change for different substrates and reactors. This value is also correlated to the loading rate of a digester, and it is one of the main parameters considered in biogas industrial plants [35].
In a BMP batch test, where daily productivity and cumulative production are monitored, it is possible to identify the optimal HRT for each biochar type tested. Optimal retention time allows maximizing biogas yield because microorganisms are maintained constantly in the exponential growth phase and that is usually lower than the duration of a BMP test (in this case 37 days) [36, 37].
The data collected show that all the different biochars produced more than the control for all the duration of the test (at day 15, 21, 25, 30, and 37).
Furthermore, the peak productivity, compared to the control at the same day, occurred differently depending on the biochar used: untreated biochars (WS, P and Pc) after 15 days, instead, oxidized biochars (WS_Ox and P_Ox) after 21 days (6 days later).
Results suggest that, for the optimization of the process in a plant using biochar as additive, HRT parameter should be shorter than usual, since the anaerobic digestion process is boosted (i.e., it is possible to produce the same amount of biogas in a shorted time).
4 Conclusions
In this study, biochars from wheat straw (WS) and poplar (P) wood were produced under different operating conditions. These biochars underwent different methodologies of oxidation to increase their cation exchange capacity (CEC). Aqueous solution oxidation was an effective method compared to dry ball-mill, providing comparable results but requiring a lower amount of reagents, leading to faster reactions and shorter sample preparation time. Oxidized wheat straw biochar (WS_Ox) and oxidized poplar biochar (P_Ox) increased their CEC by respectively + 23.1% and + 40.1% compared to their unoxidized counterparts. Both oxidized and not oxidized biochars were tested in BMP test. All the biochar-added media produced a higher amount of biogas than control (C), respectively WS + 2.2%, P + 5.9%, Pc + 4.1%, WS_Ox + 7.7%, and P_Ox + 11.3% at the end of the test (lasted for 37 days). Under the same prevailing pyrolysis conditions (technologies and process parameters used), the use of two different kinds of biochar in AD, WS, and P, showed no significant differences in terms of total biogas production. Nevertheless, WS presented greater differences among replicates.
Comparing P (char from Poplar prepared in a TGA) and Pc (char from Poplar prepared in a pilot unit) performances on yields, no significant differences in terms of biogas yields were observed, even though the two biochars were produced using the same feedstock but through different pyrolysis technologies, operating conditions and scales. This result represents a remarkable result, offering a significant economic gain in terms production costs at industrial scale, as it is demonstrates that it is possible to process the feedstock at lower temperatures, which allows to achieve a direct energy saving effect. At batch scale, oxidation proved to bincrease biogas yields, probably thanks to the enhancement of functional groups on biochar surface (CEC values). WS_Ox and P_Ox produced more biogas than all the other treatments (WS_Ox + 7.7% and P_Ox + 11.3%). Furthermore, a feedstock effect is observed in the performance of biochars: in fact, both the P (compared to WS) and P_Ox (compared to WS_Ox) showed a higher biogas production and therefore a better performance as an additive in the AD process.
The evaluation of the production peak at different days of the experiment using oxidized biochar showed the best improvement versus control (C) at the 21st day (respectively WS_Ox + 14.9% and P_Ox + 20.9%).
In conclusion, the addition of oxidized biochar (P_Ox and WS_Ox) in AD, thanks to CEC improvement of the carbonized biomass, permits to obtain a higher biogas production compared to using a biochar obtained at 400 °C maximum process pyrolysis temperatures (P and WS). However, the additional processing step necessary to oxidize the biochar needs to be further investigated as regards technical and economic feasibility at industrial scale, in view of future commercial applications.
References
Carlini M, Mosconi EM, Castellucci S et al (2017) An economical evaluation of anaerobic digestion plants fed with organic agro-industrial waste. Energies 10:1165. https://doi.org/10.3390/EN10081165
Wei X, Kusiak A (2012) Optimization of biogas production process in a wastewater treatment plant. undefined
Ariunbaatar J, Panico A, Esposito G et al (2014) Pretreatment methods to enhance anaerobic digestion of organic solid waste. Appl Energy 123:143–156. https://doi.org/10.1016/J.APENERGY.2014.02.035
Ali Shah F, Mahmood Q, Maroof Shah M et al (2014) Microbial ecology of anaerobic digesters: The key players of anaerobiosis. Sci World J. https://doi.org/10.1155/2014/183752
Fagbohungbe MO, Herbert BMJ, Hurst L, et al (2017) The challenges of anaerobic digestion and the role of biochar in optimizing anaerobic digestion. Waste Manag.
Sieber JR, McInerney MJ, Plugge CM, et al (2010) Methanogenesis: syntrophic metabolism. Handb Hydrocarb Lipid Microbiol 337–355.https://doi.org/10.1007/978-3-540-77587-4_22
Wang W, Lee DJ (2021) Direct interspecies electron transfer mechanism in enhanced methanogenesis: a mini-review. Bioresour Technol 330:124980. https://doi.org/10.1016/J.BIORTECH.2021.124980
Baek G, Rossi R, Saikaly PE, Logan BE (2021) The impact of different types of high surface area brush fibers with different electrical conductivity and biocompatibility on the rates of methane generation in anaerobic digestion. Sci Total Environ 787:147683. https://doi.org/10.1016/J.SCITOTENV.2021.147683
Liu Y, Li X, Wu S et al (2021) Enhancing anaerobic digestion process with addition of conductive materials. Chemosphere 278:130449. https://doi.org/10.1016/J.CHEMOSPHERE.2021.130449
Guo X, Sun C, Lin R et al (2020) Effects of foam nickel supplementation on anaerobic digestion: Direct interspecies electron transfer. J Hazard Mater 399:122830. https://doi.org/10.1016/J.JHAZMAT.2020.122830
Ferraro G, Pecori G, Rosi L, et al (2021) Biochar from lab-scale pyrolysis: influence of feedstock and operational temperature. Biomass Convers Biorefinery 1–11.https://doi.org/10.1007/S13399-021-01303-5/TABLES/2
Li S, Harris S, Anandhi A, Chen G (2019) Predicting biochar properties and functions based on feedstock and pyrolysis temperature: a review and data syntheses. J Clean Prod 215:890–902. https://doi.org/10.1016/J.JCLEPRO.2019.01.106
O’Laughlin J, McElligott K (2009) Biochar for environmental management: science and technology, Johannes Lehmann, Stephen M. Joseph (Eds.), Earthscan, London UK (2009), 448 p. For Policy Econ 11:535–536. https://doi.org/10.1016/J.FORPOL.2009.07.001
Wang J, Wang S (2019) Preparation, modification and environmental application of biochar: a review. J Clean Prod 227:1002–1022. https://doi.org/10.1016/J.JCLEPRO.2019.04.282
Schmidt H-P (2012) 55 Uses of Biochar. Uses Biochar Ithaka J 1:286–289
Codignole Luz F, Cordiner S, Manni A et al (2018) Biochar characteristics and early applications in anaerobic digestion-a review. J Environ Chem Eng 6:2892–2909. https://doi.org/10.1016/J.JECE.2018.04.015
Zhu X, Chen B, Zhu L, Xing B (2017) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut 227:98–115. https://doi.org/10.1016/J.ENVPOL.2017.04.032
Yuan Y, Bolan N, Prévoteau A et al (2017) Applications of biochar in redox-mediated reactions. Bioresour Technol 246:271–281. https://doi.org/10.1016/J.BIORTECH.2017.06.154
Wambugu CW, Rene ER, van de Vossenberg J et al (2019) Role of biochar in anaerobic digestion based biorefinery for food waste. Front Energy Res 7:14. https://doi.org/10.3389/FENRG.2019.00014/BIBTEX
Capson-Tojo G, Girard C, Rouez M, et al (2019) Addition of biochar and trace elements in the form of industrial FeCl3 to stabilize anaerobic digestion of food waste: dosage optimization and long-term study. J Chem Technol Biotechnol 94:.https://doi.org/10.1002/jctb.5797
Masebinu SO, Akinlabi ET, Muzenda E, Aboyade AO (2019) A review of biochar properties and their roles in mitigating challenges with anaerobic digestion. Renew. Sustain. Energy Rev. 103
Qiu L, Deng YF, Wang F, et al (2019) A review on biochar-mediated anaerobic digestion with enhanced methane recovery. Renew. Sustain. Energy Rev. 115
Pan J, Ma J, Zhai L, et al (2019) Achievements of biochar application for enhanced anaerobic digestion: a review. Bioresour. Technol. 292
Zhang L, Lim EY, Loh KC et al (2020) Biochar enhanced thermophilic anaerobic digestion of food waste: focusing on biochar particle size, microbial community analysis and pilot-scale application. Energy Convers Manag 209:112654. https://doi.org/10.1016/J.ENCONMAN.2020.112654
Fagbohungbe MO, Herbert BMJ, Hurst L et al (2016) Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour Technol 216:142–149. https://doi.org/10.1016/j.biortech.2016.04.106
Angelidaki I, Alves M, Bolzonella D et al (2009) Defining the biomethane potential (BMP) of solid organic wastes and energy crops: a proposed protocol for batch assays. Water Sci Technol 59:927–934. https://doi.org/10.2166/WST.2009.040
Rizzo AM, Pettorali M, Nistri R, Chiaramonti D (2019) Mass and energy balances of an autothermal pilot carbonization unit. Biomass Bioenerg 120:144–155. https://doi.org/10.1016/J.BIOMBIOE.2018.11.009
Shrotri A, Kobayashi H, Fukuoka A (2016) Mechanochemical synthesis of a carboxylated carbon catalyst and its application in cellulose hydrolysis. ChemCatChem 8:1059–1064. https://doi.org/10.1002/CCTC.201501422
Madduri S, Elsayed I, Hassan EB (2020) Novel oxone treated hydrochar for the removal of Pb(II) and methylene blue (MB) dye from aqueous solutions. Chemosphere 260:127683. https://doi.org/10.1016/J.CHEMOSPHERE.2020.127683
Wu D, Zhang Z, Yu Z, Zhu L (2018) Optimization of F/M ratio for stability of aerobic granular process via quantitative sludge discharge. Bioresour Technol 252:150–156. https://doi.org/10.1016/J.BIORTECH.2017.12.094
Bazzechi Pietro (2020) Use of tailor-made biochar in anaerobic digestion: energy, technical and economic analysis. Dept. of Industrial Engineering (DIEF). Università degli Studi di Firenze
Buswell AM, Mueller HF (2002) Mechanism of methane fermentation. Ind Eng Chem 44:550–552. https://doi.org/10.1021/IE50507A033
Chynoweth DP, Turick CE, Owens JM et al (1993) Biochemical methane potential of biomass and waste feedstocks. Biomass Bioenerg 5:95–111. https://doi.org/10.1016/0961-9534(93)90010-2
Sun J, Lian F, Liu Z et al (2014) Biochars derived from various crop straws: characterization and Cd(II) removal potential. Ecotoxicol Environ Saf 106:226–231. https://doi.org/10.1016/J.ECOENV.2014.04.042
Meegoda JN, Li B, Patel K, Wang LB (2018) A review of the processes, parameters, and optimization of anaerobic digestion. Int J Environ Res Public Health 15:.https://doi.org/10.3390/IJERPH15102224
Shi XS, Dong JJ, Yu JH et al (2017) Effect of hydraulic retention time on anaerobic digestion of wheat straw in the semicontinuous continuous stirred-tank reactors. Biomed Res Int. https://doi.org/10.1155/2017/2457805
Van DP, Fujiwara T, Tho BL, et al (2020) A review of anaerobic digestion systems for biodegradable waste: configurations, operating parameters, and current trends. Environ. Eng. Res. 25
Acknowledgements
The authors also wish to acknowledge Giulia Lotti and Pietro Bazzechi from RE-CORD lab for the analytical work on feedstocks and products, as well as on BMP tests.
Funding
Open access funding provided by Politecnico di Torino within the CRUI-CARE Agreement. Benedetta Ciuffi wishes to thank Fondazione Cassa di Risparmio Firenze for the grants related to the project 2018–0930 “PIRACY”.
Author information
Authors and Affiliations
Contributions
Conceptualization: Luca Rosi, David Chiaramonti; methodology: Luca Rosi; formal analysis: Marco Cenni, Benedetta Ciuffi, David Casini; investigation: Luca Rosi, Marco Cenni; data curation: Benedetta Ciuffi, David Casini; writing—original draft preparation: Benedetta Ciuffi, Marco Cenni; writing—review and editing: David Casini, Andrea Maria Rizzo, Luca Rosi, David Chiaramonti; supervision: David Chiaramonti, Luca Rosi; project administration: David Chiaramonti; resources: David Chiaramonti. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rosi, L., Cenni, M., Ciuffi, B. et al. Enhancing biogas production in anaerobic digestion by the addition of oxidized and non-oxidized biochars. Biomass Conv. Bioref. 14, 5457–5468 (2024). https://doi.org/10.1007/s13399-022-02813-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02813-6