Abstract
One of the challenges in biofuel production from lignocellulosic wastes is to improve its conversion to solvents; therefore, new strategies to enhance xylose uptake are required due to be the secondary abundant sugar. In this context, a novel fermentation strategy integrating a co-culture of Clostridium acetobutylicum and Saccharomyces cerevisiae with pH control was developed. Initially, two different buffers, ammonium acetate and calcium carbonate, were tested under pHmin > 4.8 by fermenting 60 g L−1 of glucose with the C. acetobutylicum monoculture. Ammonium acetate was selected for fermenting media as butanol production was increased from 9.8 to 10.9 g L−1 over the calcium carbonate test. Comparing with the spontaneous acetone-butanol-ethanol (ABE) fermentation with C. acetobutylicum when no xylose consumption was observed, xylose consumption was efficiently increased by controlling pHmin > 4.8. The xylose consumption was > 47% either by using a 45:15 g L−1 glucose:xylose mixture or with rice straw (RS) hydrolysate. Clostridium monoculture using RS hydrolysate and pHmin > 4.8 produced a butanol (ABE) concentration of 6.5 (9.5) g L−1. While it increased to 7.0 (13.1) g L−1 when the co-culture with S. cerevisiae was used using same pH regulation strategy mainly due to ethanol increase up to 2.7 g L−1. Moreover, the xylose uptake doubled to 94% due to amino-acid secretion by yeast. Overall, this combined strategy was a very effective method for promoting sugar consumption and ABE solvent production from lignocellulosic waste.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The world is moving towards a more sustainable economy based on the use of renewable sources urged, among other factors, by the climate change awareness and the necessity of limiting the greenhouse gas emission [1]. Butanol is a highly appreciated biofuel due to its physicochemical properties and would not require extensive investments as it can be delivered via current infrastructure [2,3,4]. Biobutanol can be produced via acetone-butanol-ethanol (ABE) fermentation by solventogenic Clostridia. However, ABE fermentation has downsides as low concentrations or substrate costs. First-generation biobutanol was produced by sugar- or starch-based feedstock (like sugarcane or corn) with drawbacks as high cost of raw materials and competition in the food supply. Thus, the lignocellulosic biomasses from agro-food activities have grown in interest due to low cost and wide availability [5]. Lignocellulosic biomasses, such as rice straw (RS), require a pretreatment prior to sugar release from cellulose and hemicellulose after enzymatic hydrolysis. Among the pretreatments, alkalis are capable of swelling the cellulose structure and remove acetyl groups, lignin and uronic acid substitutions, hence increasing enzyme accessibility to the polysaccharides [6]. From a compendium of 77 hydrolyzed lignocellulosic feedstock, Birgen et al. [7] obtained a median value of 23.6 and 10.8 g L−1 of glucose and xylose (glucose:xylose ratio of ~ 2.2:1); thus, xylose is the secondary monosaccharide in hydrolysate composition. However, the carbon catabolite repression (CCR) of glucose over xylose hinders the overall efficiency of ABE fermentation [8], being one of the drawbacks when using lignocellulosic biomass.
Regarding solventogenic Clostridium species, it was reported that Clostridium beijerinckii is capable of uptake xylose efficiently in presence of glucose [9], which could be due to the presence a big gene cluster of D-xylose pathway genes found in some strains such as C. beijerinckii NCIMB 8052 [10]. In the case of Clostridium acetobutylicum, previous studies reported that the xylose catabolic routes, pentose phosphate [11] or the phosphoketolase pathway, are influenced by the level of xylose present in the media [12]. Thus, more efforts should be made to elucidate strategies to better control the xylose uptake. This is especially important in the case of glucose:xylose mixtures to avoid CCR, among the singular characteristics which make the study of C. acetobutylicum of interest highlights its capability to form a denser biofilm due to cell to cell communication [13], which could led to the increase the overall productivity by promoting cell immobilization [14].
In addition, the media pH has been shown to play an important role on the fermentation profile of C. acetobutylicum. For example, controlling the minimum pH on batch fermentation boosted glucose consumption and butanol production by alleviating acid crash, with an increment from 7.47 to 11.22 g L−1 butanol when comparing with no pH control [15]. Regulation of pH was also successfully implemented in continuous reactors with C. acetobutylicum using lignocellulosic substrates [16]. Moreover, pH-controlled continuous fermentations have been also shown effective with xylose [17, 18]. Another strategy that has been shown efficient to improve sugar uptake and solvent production by C. acetobutylicum was formulation of the media with CaCO3 producing 9.65 g L−1 of butanol from a glucose:xylose mixture (30:30 g L−1) with 82% of sugar consumption [19]. Indeed, CaCO3 was demonstrated to reduce the residual xylose with glucose:xylose mixtures regardless of the monosaccharide ratio in ABE fermentation [20]. Jiang et al. [21] also demonstrated that pH control could increment xylose uptake despite their sugar ratio. Best results were achieved at the lower glucose:xylose ratio tested (1.5:1); consumption of xylose was enhanced from 11.6% without pH control to 66.1% by controlling pH after the acidogenic phase. Therefore, it seems that pH regulation independently of the implemented strategy (control pH or media buffering) could improve the xylose consumption in presence of glucose.
As a considerable fermentation strategy, co-culture of two or more microorganisms has been applied in several bioprocesses to confront the limitations of pure strains, like biofuels or food industries [22]. Some examples are the consolidated bioprocessing (CBP) to combine cellulolytic bacteria and another microorganisms, such as lactic acid bacteria [23] or the co-culture with amylolytic and ethanol fermentation microorganisms to develop competitive simultaneous saccharification and fermentation (SSF) [24, 25]. Recently, co-culture of S. cerevisiae and Clostridium species have gained interest. The metabolic abilities of the yeast could contribute to increase monosaccharide uptake and biofuel production (butanol and ethanol) at the co-culture with Clostridium species. The better outcome of the fermentation may be due to the yeast ability to secrete amino acids to the fermentation media [26, 27]. Some studies have shown that co-culture of S. cerevisiae and C. acetobutylicum improves butanol production from starch-based media [27, 28], although its application to lignocellulosic waste remains unexplored. For instance, Qi et al. [29] were able to increase ABE production from 17.66 to 42.56 g L−1 by using a co-culture with S. cerevisiae and C. acetobutylicum CH02 over the Clostridium monoculture when 150 g L−1 of cassava was fermented.
The main aim of this work was to evaluate the combination of pH regulation and a co-culture system of C. acetobutylicum and S. cerevisiae with the target to improve solvent concentrations and sugar uptake from RS hydrolysate. Initially, it was evaluated the effect of the pH control in the exploitation of model substrates (glucose and xylose) by C. acetobutylicum for butanol production by using two buffering components (acetate and carbonate). The effect of the best buffering component was further tested with hydrolysates from alkaline-pretreated RS in order to define the pH regulation strategy. Once pH regulation was established, the co-culture system of S. cerevisiae and C. acetobutylicum was studied by using model substrates. The overall strategy was further validated by using alkaline-pretreated RS hydrolysates. This work is expected to contribute to enhance solvent concentrations and sugar consumption in fermentations from lignocellulosic biomass.
2 Material and methods
2.1 Microorganisms, fermentation media, and chemical reagents
C. acetobutylicum DSM 792 was purchased from DSMZ, Germany (German Collection of Microorganisms and Cell Cultures). The culture was stored in 20% glycerol at − 80 °C. Prior to the experiments, the microorganism was cultured statically at 37° in 19 g L−1 of Reinforced Clostridial Medium (RCM) fortified with 10 g L−1 of glucose as seed inoculum. S. cerevisiae EYS4 was maintained on YPD agar (yeast extract, 10 g L−1, peptone 20 g L−1, glucose 20 g L−1, and agar 20 g L−1) at 4 °C. The yeast seed culture was inoculated into 50 ml of YPD broth and incubated at 37 °C and 150 rpm. The fermentation medium composition was (g L−1): sugars (glucose, xylose, or hydrolysate); yeast extract, 5; K2HPO4, 0.5; KH2PO4, 0.5; NH4Cl, 2; MgSO4.7H2O, 0.2; MnSO4.7H2O, 0.01; FeSO4.7H2O, 0.05; resazurin sodium salt, 0.001; and antifoam 204, 0.01%. Two different media formulations were tested. Acetate buffer (CH3COONH4; 2.2 g L−1) replaced the NH4Cl of the fermentation medium. Carbonate buffer was formulated by adding CaCO3 (2.5 g L−1) to the above-mentioned fermentation media. The media was sterilized by autoclave at 121 °C during 20 min and the metal solution was filter-sterilized by 0.22 μm. Chemicals were obtained from VWR, except for antifoam 204 (Sigma-Aldrich), CaCO3 (Merck), and yeast extract (Alfa Aesar).
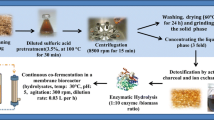
2.2 RS pretreatment and hydrolysis
The biomass was obtained from local farmers of L’Albufera situated close to Valencia (Spain). The untreated RS composition on dry weight basis was 35.8 ± 2.1% of cellulose, 17.5 ± 1.4% of hemicellulose, 0.1 ± 0.0% of acid soluble lignin, 14.3 ± 0.4% of acid insoluble lignin, 16.7 ± 0.1% of ash, and 15.6% of others. Prior optimized from a previous study [30], the following conditions were applied to obtain the hydrolysate: dried RS was milled to 0.1- to 2-mm particle size; it was pretreated with 0.75% NaOH and a solid loading of 5% (w/w) at 134 °C for 40 min in an autoclave (MED20, J.P. Selecta, Spain), then dried in an oven at 45 °C for 24 h prior storage at 4 °C. Enzymatic hydrolysis was performed with 8% (w/w) solids loading at pH 5.2 with a concentration of 20 FPU g-dw−1 (Cellic® CTec2, Novozyme, Denmark) at 50 °C and 150 rpm for 72 h in an orbital incubator (G25, New Brunswick Scientific, USA). The hydrolysate was stored at − 4 °C prior use.
2.3 Experimental setup
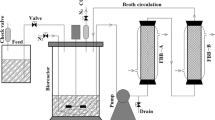
2.3.1 Monoculture and co-culture reactors
The reactor fermentations were performed with a working volume of 0.8 L (total volume of 1.1 L). The media was flushed with nitrogen gas for 30–35 min before inoculation. A 5% v/v inoculum of C. acetobutylicum was used in each experiment. The fermentations were performed at 37 °C and 120 rpm. For the co-culture experiments, a 5% v/v inoculum of S. cerevisiae was used. Two kinds of experiments without and with pH control were performed. A minimum set-point control was carried out with NaOH (3 M) to keep the pH above the threshold. The experiments were followed using a Tris-compatible flat pH sensor with LoggerPro software (Vernier, USA). The pH probes were sterilized following the procedure by Qureshi et al. [31]. They were sterilized by submerging them in a 50% ethanol solution (v/v) for 12–24 h. After which, the probes were washed with sterile water. Samples were taken at appropriate time points to analyze cell growth, sugars, and products (butyric acid, acetic acid, butanol, acetone, and ethanol).
2.3.2 Co-culture pre-screening
Fermentations were performed in 50-mL serum bottles with 40 mL of working volume; the inoculation was carried out with 5% v/v of C. acetobutylicum and then 5% v/v of S. cerevisiae at three time lags (0, 5, and 10 h). Monocultures of C. acetobutylicum and S. cerevisiae were performed as controls. Anaerobic conditions were obtained by sparging nitrogen in the fermentation medium. The fermentations were carried out in an orbital incubator at 37 °C and 150 rpm by duplicate. Butanol production was selected as the criteria for establishing the inoculation procedure of the co-culture reactors.
2.4 Experimental plan
Five sets (runs) of experiments were carried out to develop a combined regulation pH strategy with a co-culture fermentation of C. acetobutylicum and S. cerevisiae. All runs were performed without and with pH control. The value of the minimum pH (4.8) was selected from a prior study by using the same glucose concentration and 5 g L−1 carbonate [15]. Run 1 was performed with 60 g L−1 of glucose to determine the best buffer composition for the two formulations. From these results, ammonium acetate was selected as the best buffer alternative. For the rest of the experiments, two pH regulations were employed: (1) ammonium acetate dosage or (2) ammonium acetate dosage combined with minimum NaOH pH control. The effect of the pH regulation on xylose in presence of glucose was assessed (run 2). Run 3 was performed by replacing the synthetic substrate with alkali-pretreated RS as described in Sect. 2.2. In run 4, a model concept of co-culture was developed using a synthetic mixture of 35:15 glucose:xylose mimicking RS hydrolysate. In this case, S. cerevisiae was inoculated at a selected time after C. acetobutylicum according to results derived from Sect. 3.2.1. Once the co-culture model was established, the effect on solvent production was checked by using RS hydrolysate (run 5). Runs 3 to 5 were performed by duplicate.
2.5 Analytical methods
The fermentations were monitored by sampling at desired time points. Cell biomass was determined using an UV–Vis spectrophotometer (SpectroFlex 6600, WTW) at 600 nm (OD600). Biomass concentration of C. acetobutylicum (gDM L−1) was estimated by using gDM L−1 = 0.2941·OD600 + 0.0331 (R2 = 0.9908). Samples were centrifuged at 10,000 rpm for 5 min (MEGA Star 3.0, VWR, Germany) and filtered by 0.22 μm for analysis. Sugars and products were determined by liquid chromatography (Agilent 1100 Series HPLC system, Agilent Technologies, USA) using a refractive index detector (RID) and diode array detector (DAD) with an Aminex® HPX-87H column (300 mm × 7.8 mm, Bio-Rad Laboratories Inc., USA). The system was operated at 50 °C. A refractive index detector (RID) was used to detect sugars, butanol, and ethanol, while a diode array detector (DAD) was employed at 210 nm to detect acetic, butyric, and levulinic acids and at 280 nm to detect acetone, furfural, and 5-(hydroxymethyl)furfural (5-HMF). The mobile phase was 5 mM of sulfuric acid with a flow of 0.6 mL min−1. The RID was kept at 35 °C. The running time of the analysis was 45 min. The Folin-Denis method was used to quantify the total phenolic compounds expressed as gallic acid equivalents [32]. The values of pKa of acetic acid (4.76) and butyric acid (4.82) were used with the Henderson-Hasselbalch equation (Eq. (1)) to obtain the concentration of undissociated acids.
The glucose consumption rate was estimated at the exponential growth phase by Eq. (2):
where − qglucose corresponds to the glucose consumption rate (g L−1 h−1), St1 and St2 are the monosaccharide concentrations at the starting and ending point of the exponential growth phase (g L−1), and t2 and t1 are the times at the beginning and end of the exponential growth phase (h).
3 Results and discussion
3.1 Solvent production from RS hydrolysate by C. acetobutylicum
3.1.1 Effect of the media buffer using glucose as model substrate
The influence of the buffer species on butanol production from glucose (60 g L−1) as main substrate in RS hydrolysate was evaluated by using two alternative components, CH3COONH4 or CaCO3, added to the buffering phosphate species (run 1). Main representative parameters of the ABE fermentation experiments without and with pH control (pHmin > 4.8) are summarized in Table 1. As it can be seen, the reactors without pH control showed a different behavior depending on the buffer compound. A butanol production of 11.2 g L−1 was achieved when acetate was used but only 3.2 g L−1 was obtained with carbonate. ABE production also differed, being 19.2 g L−1 with acetate and 5.9 g L−1 with carbonate. The substantial difference in solvent production with acetate compared with carbonate was related to the glucose consumption in both reactors. In that sense, the glucose was completely depleted (in less than 50 h) with acetate while the carbonate reactor only consumed 55% of the reducing sugar. Therefore, higher butanol yield was also observed (0.199 g g−1 for acetate and 0.093 g g−1 for carbonate). The pH recovery was higher for acetate (4.18 to 4.84) than for carbonate (4.57 to 4.71), which was connected to a better development of ABE fermentation when using acetate instead of carbonate. The nearly double glucose consumption rate with carbonate (− qglucose: 1.80 g L−1 h−1) seemed to impact adversely on the solventogenesis. Indeed, the level of CaCO3 (2.5 g L−1) was not sufficient to prevent the acid crash phenomenon. The total undissociated acid species were higher than 60 mM, the referenced threshold for Clostridium species [33]. The lower performance of the reactor with carbonate over the reactor with acetate could be explained by the differences in the maximum butyric acid concentration. The production of acids can be modulated by changing the fermentation media; for example, calcium carbonate can enhance the production of acids in ABE fermentation. Ren et al. [8] observed an increment of ~ 1.3-fold when fermenting with P2 media over P2 media supplemented with calcium carbonate with C. acetobutylicum ATCC 824. Similarly, the reactor with acetate reached 2.87 g L−1 of butyric acid (Fig. 1a), while the reactor with carbonate reached 4.88 g L−1 (data not shown), thus showing an ~ 1.7-fold increase when the calcium carbonate is used to buffer the fermentation. These results indicated the effect of buffer media formulation on C. acetobutylicum DSM 792 metabolism. In this way, Luo et al. [34] obtained 1.2 g L−1 of butanol when fermenting with 0.75 g of K2HPO4 and KH2PO4 as media buffer, showing that the usage of sole phosphate species as buffer failed to produce enough butanol. Higher level of carbonate (5 g L−1) allowed to achieve better glucose consumption without pH control [15, 19], although acid crash was reported in some extent [15].
The pH regulation had an impact on the switch between acidogenesis and solventogenesis of C. acetobutylicum DSM 792 independently of the media buffer. For the two assays, the pH regulation was activated at early times (6–7 h) when acids production occurred, thus allowing a good pH recovery in both reactors after exponential growth phase ended, with final pHs > 5.3. Respecting the reactor with the acetate buffer, the final butanol concentration was very similar to the uncontrolled counterpart (10.9 over 11.2 g L−1) despite the reduction in ABE solvents (16.2 over 19.2 g L−1). In this case, ABE composition shifted to lower acetone proportion (6.1:3.2:0.7 butanol:acetone:ethanol), closer to the theoretical ratio (6:3:1), compared with the non-pH control reactors (acetate: 5.1:3.8:1.0; carbonate: 4.6:3.7:1.7). Furthermore, butanol yields were similar (> 0.18 g g−1), showing that no butanol inhibition occurred when using acetate buffer without or with minimum pH control. Interestingly, the glucose consumption rate with ammonium acetate increased in ~ 2.8-fold with pH regulation (2.67 g L−1 h−1), being the highest achieved over all the experiments. The fast sugar consumption rate shortened the fermentation time from ~ 50 to ~ 30 h. Therefore, a substantially increase in butanol productivity was achieved. The pH control enhanced glucose consumption rate with other Clostridium species such as C. beijerinckii IB4. A higher glucose consumption rate (1.67 ± 0.05 g L−1 h−1) was achieved when pH was controlled (pH at 5.5 after reached) versus no pH control (1.03 ± 0.04 g L−1 h−1) [35]. In the case of using carbonate, the pH regulation impacted favorably on the solvent production and glucose depletion, but not in the glucose consumption rate. The complete depletion of glucose led to an increment in butanol (ABE) production from 3.2 (5.9) to 9.8 (15.1) g L−1 (Table 1), while butanol yield (0.172 g g−1) was similar to that of the fermenter with acetate buffer. Furthermore, pH regulation at early stages was shown as a very efficient strategy to avoid acid crash comparing with making changes on media formulation. Moreover, Jiang et al. [35] observed that delaying the pH control more over 24 h of the fermentation did not avoid acid crash. Our approach, based on fixing a minimum pH rather than establishing a specific time to start pH control, allows well-fitting the pH regulation during the exponential growth phase independently of the growth kinetics. In our case, both buffers exhibited good performance in solvent production when active pH control was used. The main difference between them was in the glucose rate, being 40% higher in the case of using acetate. Consequently, the higher glucose consumption rate with ammonium acetate led to a greater productivity over the use of calcium carbonate while reducing the fermentation time. Moreover, without pH regulation the use of 2.2 g L−1 of ammonium acetate does not exhibit acid crash leading to better solvent production than 2.5 g L−1 of calcium carbonate. Thus, by the criteria of incrementing the solvent productivity (with pH regulation) or overcome acid crash (without pH regulation), subsequent experiments were carried out with ammonium acetate as media buffer.
3.1.2 Effect of the pH regulation on xylose and mixture of glucose:xylose
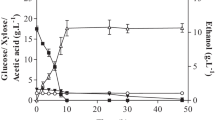
In order to evaluate the exploitation of lignocellulosic wastes onto ABE solvents, the effect of the pH regulation on xylose (secondary reducing sugar) consumption under the presence of glucose (primary reducing sugar) was studied. Two sets of experiments were performed by keeping constant the reducing sugar concentration at 60 g L−1 and in absence or presence of glucose (run 2). The selected mixture was of 45:15 g L−1 glucose:xylose, which is on the typical proportion between the two monosaccharides in hydrolysates from lignocellulosic waste. Fermentation profiles with uncontrolled pH and controlled pH using xylose and with a mixture of glucose:xylose were compiled in Fig. 1c-f. For comparison purposes, patterns with pure glucose and same media formulation (2.2 g L−1 acetate; Table 1) were also depicted (Fig. 1a, b). From the reactors with pure xylose (Fig. 1c, d), it can be seen a lag phase of approximately 24 h not observed previously with glucose (Fig. 1a, b). This is linked to the change of the carbon source from the inoculum growth (RCM with glucose). Without pH control, similar sugar consumption rates were achieved during exponential growth phase, independently of the monosaccharide (glucose: 0.96; xylose: 1.13 g L−1 h−1). Nevertheless, xylose consumption declined from 48 h when butanol concentration started to increase (Fig. 1c) while no changes on consumption were observed with glucose. This reduction in xylose uptake would be related to product inhibition. In this sense, a butanol concentration of ~ 8.0 g L−1 had been reported as inhibitory when using xylose as carbon source [36]. At the end of the fermentation, only 72% of xylose was consumed, whereas butanol production reached the inhibitory value (8.0 g L−1; butanol yield of 0.181 g g−1). On the other hand, the fermentation of the sugar mixture (45:15) without pH control stopped at 24 h due to an acid crash phenomenon (Fig. 1e). Nevertheless, a butanol (ABE) production of 4.9 (7.7) g L−1 was achieved from a glucose consumption of 63%, with a butanol (ABE) yield of 0.182 (0.289) g g−1. Glucose consumption rate, with a 25% lower initial glucose level, was faster in the mixture (1.35 g L−1 h−1) than with pure glucose (0.96 g L−1 h−1), however was not sufficiently high to promote the solventogenesis shift before acid crash occurred. Results indicate that a minimum level of initial glucose would be required for completion of ABE fermentation with this strain when pH evolved spontaneously.
A similar xylose consumption rate was observed with and without pH control; however, the pH control caused a carbon flux redistribution to boost biomass production (Fig. 1d). A OD600 ~ 1.6-fold higher was achieved (at the maximum point) when using pure xylose comparing with the use of glucose as carbon source (xylose: 24.2 or 7.2 g L−1 at ~ 62 h; glucose: 14.9 or 4.4 g L−1 at ~ 26 h). We speculate that the metabolism shift towards cell synthesis was associated to the availability of organic nitrogen from yeast extract (5 g L−1). W. Jiang et al. [21] observed some increase in biomass growth with C. acetobutylicum ATCC 824 under pH control after the acidogenic phase with same yeast extract concentration, which was accompanied by an improvement of solvent production. However, contrarily to them we observed a decrease in butanol (ABE) production from 8.0 (13.1) g L−1 without pH control to 5.3 (8.2) g L−1 with pH control at 4.8 (Fig. 1c, d), probably due to the excessive biomass growth achieved in our study. In any case, data have shown that xylose will be consumed after glucose exhaustion when using lignocellulosic hydrolysates. In the sugar mixture experiment, 60 h was required to start xylose consumption after glucose depletion. In this regard, the utilization of pH control with the glucose:xylose mixture allowed avoiding acid crash, thus enhancing the full glucose conversion in < 24 h (− qglucose: 2.38 g L−1 h−1), and favoring the further xylose uptake without an excessive biomass growth (Fig. 1f). Although the pH regulation was unable to avoid the CCR phenomena, positively this delay on xylose consumption did not adversely impact on the butanol (ABE) production, which was 8.8 (13.2) g L−1. Indeed, solvent production was quite close to the estimated values from the experimental yields with solely monosaccharides under pH control (butanol: 9.3; ABE: 13.9 g L−1). As conclusion, ABE fermentation with C. acetobutylicum DSM 792 benefits for pH control to efficiently produced ABE solvents from glucose and xylose mixtures. Moreover, the implementation of in-situ product recovery techniques would improve even more the xylose uptake in presence of glucose by avoiding butanol inhibitory levels.
3.1.3 The pH validation strategy using RS hydrolysates
The pH regulation strategy was validated by replacing the model substrates by the alkali-pretreated RS hydrolysate (run 3; Fig. 2). The hydrolysate characteristics at the final medium can be observed in Table 2. At the beginning of the fermentation reducing sugar concentration was 34.0 ± 0.7 g L−1 of glucose and 14.0 ± 0.6 g L−1 of xylose. In addition, the initial acetic acid was 4.9 ± 0.7 g L−1. The cellobiose and arabinose remained nearly unchanged through the experiment. The total concentration of phenolic compounds was 0.13 ± 0.01 g L−1. It was expected from the assay with the mixture model (Fig. 1e) that the lack of pH control caused an acid crash stopping the fermentation at 24 h (Fig. 2a), hence corroborating that low initial levels of glucose require of pH control for ABE fermentation with this strain. Under pH control, a butanol (ABE) production of 6.5 ± 0.1 (9.5 ± 0.8) g L−1 was achieved at the end of the fermentation. The pH control favored the consumption of xylose after glucose was depleted (47 ± 10%). Some xylose remaining as final butanol approached to inhibitory levels. In addition, butanol and ABE yields (butanol: 0.167 ± 0.005, ABE: 0.261 ± 0.002 g g−1) were slightly better over the synthetic mixture (butanol: 0.161, ABE: 0.243 g g−1). The complex composition of the RS hydrolysate better employed the sugar content and mitigated the CCR phenomena when the pH control was applied. In fact, the butanol (ABE) predicted concentrations from the results obtained with the synthetic media were 7.0 (10.5) g L−1, similar to the experimental productions obtained in the assay (6.5 (9.5) g L−1), thus validating the pH control strategy in terms of solvent production. The glucose consumption rates were 0.96 ± 0.13 and 2.38 ± 0.02 g L−1 h−1 for no pH and pH-controlled fermentations, respectively. Similar values were obtained with synthetic (pure glucose and sugar mixture). Hence, demonstrating no inhibition of glucose uptake occurred when the RS hydrolysate is fermented. The production herein obtained are among the referenced concentrations. For example, Amiri et al. [37] produced 7.1 (10.5) g L−1 of butanol (ABE) by using organosolv-pretreated RS with C. acetobutylicum NRRL B-591. Other Clostridium species can be also used; in this sense, Valles et al. [30] achieved a higher butanol (ABE) concentration of 10.1 (16.7) g L−1 with alkali-pretreated RS using C. beijerinckii DSM 6422.
3.2 Solvent production by co-culture of C. acetobutylicum and S. cerevisiae
3.2.1 Effect of time inoculation
Prior to evaluation of the effect of pH regulation on the co-culture of C. acetobutylicum DSM 792 and S. cerevisiae EYS4, it was established the elapse time between inoculations of both species to promote butanol production. A screening experiment with 50-mL serum bottles was carried out by inoculating S. cerevisiae at 0, 5, and 10 h after C. acetobutylicum inoculation. Initial glucose and xylose levels mimicked the RS hydrolysate concentration (35 and 15 g L−1 of glucose and xylose). To analyze the effect of inoculation time on the substrate competition between the two species, and hence on the solvent redistribution, solvent production at early stage (24 h) was considered. Solvent production and glucose consumption rate at 24 h of fermentation along with the remaining glucose at 10 h were summarized in Table 3. The butanol production at 24 h increased in all co-culture experiments compared with the Clostridium monoculture (Table 3). Moreover, ABE production increased due to ethanol production by S. cerevisiae. In fact, the inoculation of the yeast simultaneously to C. acetobutylicum (0 h) led to mainly ethanol synthesis (ethanol/butanol ratio: 3.4 ± 0.1). The earlier presence of the yeast (0 h) caused a very early competition from the available sugar in the fermentation media between the two species, adversely impacting on butanol production. By postponing 5 h its inoculation the ethanol/butanol ratio decreased drastically (ethanol/butanol ratio: 1.1 ± 0.1), indicating that the conversion of glucose to acids by Clostridium was not hindered by S. cerevisiae growth. Further delay on the yeast inoculation (10 h) did not improve butanol proportion (ethanol/butanol ratio: 1.2 ± 0.2) while decreasing the overall glucose consumption rate. Although yeast inoculation time at 0 h gave better results in terms of ABE production, it was decided to delay 5 h the inoculation time of the yeast from the bacteria inoculation to promote butanol production over ethanol.
3.2.2 Effect of pH regulation on the co-culture
Batch reactors without pH control and by controlling pHmin > 4.8 were performed for co-culture fermentations using C. acetobutylicum and S. cerevisiae. The inoculation of S. cerevisiae was performed at 5 h after the beginning of the fermentation as indicated previously. The composition of the synthetic media (glucose: 35, xylose: 15 g L−1; run 4) was equal to the concentration of the major sugars on the alkali-pretreated RS hydrolysate (Table 2), having a slightly lower content in glucose than the synthetic mixture used in previous experiments with the solely Clostridium strain (glucose: 45, xylose: 15 g L−1). The co-culture fermentation patterns are depicted in Fig. 3. Regarding the uncontrolled pH experiment (Fig. 3a), it was observed the same acid crash phenomenon as without the use of S. cerevisiae (Fig. 1e); the pH decreased rapidly to 4.0, so glucose was not completely exhausted. However, a higher ABE production was achieved (9.3 ± 0.2 g L−1 g L−1 versus 7.7 g L−1), indicating the beneficial effect by the presence of the yeast to improve the overall glucose uptake (from 66 to 83 ± 5%), even if an early acid crash occurred. Glucose consumption rate was also higher (1.65 ± 0.04 g L−1 h−1) compared to the monoculture (1.35 g L−1 h−1). The increment in solvents was mainly due to the increase in ethanol production by the alcoholic fermentation of S. cerevisiae (from 0.3 to 3.4 g L−1). Butanol production versus total available glucose was only slightly higher in presence of the yeast than in its absence, as 4.0 ± 0.6 g L−1 of butanol with 35 g L−1 of glucose for the co-culture was obtained compared with 4.9 g L−1 of butanol with 45 g L−1 for the monoculture. In any case, these results showed that independently of the presence of the yeast, pH regulation is required for a properly development of the C. acetobutylicum metabolism.
The profiles of sugar consumption and acid and solvent production during the co-culture fermentation under pH control are shown in Fig. 3b. The total solvent production was 10.9 ± 0.6 g L−1, from which butanol was 6.2 ± 0.8 g L−1. Unsuccessfully, ABE production versus total available sugar was equal to that observed with the solely Clostridium assay. Nevertheless, ABE composition slightly shifted to more ethanol ratio due to the competition of both species for the glucose. The reduction on butanol production considering the potential production if all sugars were metabolized by the Clostridium strain was of 1.2 g L−1. Qi et al. [38] tested several combinations of elapsing times between inoculation of C. acetobutylicum and S. cerevisiae by using a mixture of 25:25 glucose:xylose. As we also observed, test butanol concentration was not improved in any of the co-culture assays comparing with the mono-Clostridium test. The best results in terms of ABE production obtained by these authors corresponded to the initial inoculation of S. cerevisiae followed by inoculation 24 h after C. acetobutylicum. By using this strategy, these authors slightly improved ABE production from 18.45 g L−1 (solely C. acetobutylicum) to 19.62 g L−1. However, a drastic reduction in butanol production (5.29 vs 11.22 g L−1) was given due to the faster metabolic rate of the yeast. In terms of butanol production, the delay in the yeast inoculation seems a better strategy.
Although the presence of the yeast did not reduce the adaptation time of the Clostridium strain to start xylose consumption, it enhanced the xylose consumption up to 85 ± 13%. Positively, it was accompanied by a simultaneously increase of butanol up to 2.4 ± 0.8 g L−1 and with some acetone production (0.8 ± 0.2 g L−1). The beneficial effect on the conversion of xylose to butanol by C. acetobutylicum would be linked to the ability of S. cerevisiae to secrete amino acids which could be uptake by the Clostridium species. In this regard, Wu et al. [26] monitored the secreted amino acids (aspartic, aliphatic, and aromatic acid family and arginine) by S. cerevisiae in a co-culture system with C. beijerinckii, observing, for example, an increment of 150% for phenylalanine concentration over the Clostridium monoculture. The assimilation of amino acids by Clostridium species would increase monosaccharide intracellular transportation and butanol tolerance [26, 27]. This evidence opened to test the co-culture system in more realistic conditions.
The profiles of sugar utilization and product formation during the co-culture fermentation of alkali-pretreated RS hydrolysate (run 5) are shown in Fig. 4. As in the previous experiments occurred, acid crash impeded an adequate development of the solventogenesis under lack of pH control (Fig. 4a), corroborating the importance of controlling pH in such system. By controlling the pH, it was achieved the highest solvent production among the experiments with the same initial sugar levels (ABE: 13.1 ± 0.1, from which butanol: 7.0 ± 0.4, acetone: 3.4 ± 0.2, and ethanol: 2.7 ± 0.7 g L−1). Successfully, the S. cerevisiae and C. acetobutylicum co-culture was able to increase not only ABE, but also slightly the butanol production. The enhancement of the ethanol production (~ 3.0-fold) in this co-culture fermentation is of importance for the better exploitation of the RS hydrolysate. The use of alkali RS hydrolysate in the co-culture alleviated in some extent the lag phase of the xylose metabolism, as the consumption started at ~ 72 h instead of ~ 120 h. At the end of the fermentation, the maximum xylose uptake (94 ± 1%) was detected, which was ~ 2.0-fold over the RS hydrolysate monoculture. At the same time, ABE production increased suddenly up to 4.4 ± 0.1 (butanol: 2.7 ± 0.2, acetone: 1.3 ± 0.0, ethanol: 0.4 ± 0.3) g L−1. The better results in comparison with the synthetic media can be associated to the complex chemical composition of the RS hydrolysate. In this sense, Jin et al. [39] observed a faster sugar consumption when fermenting apple pomace residue compared with using a sugar solution; furthermore, Moradi et al. [40] produced more butanol when fermenting with alkali-pretreated RS hydrolysate over a pure sugar medium. Literature regarding the utilization of co-cultures with Clostridium and S. cerevisiae is still scarce and most of them used starchy substrates (Table 4). In our case, the combination of an adequate pH regulation and the co-culture system with RS hydrolysate substantially boosted the consumption of xylose, which allowed the better use of the substrate (94% over the 47% of the monoculture). This led to an increase on ABE production of about 38% (Table 4). The ABE yield remained similar due to simultaneous increment in ABE production and sugar consumption. Maximum ABE productivity remained constant over the Clostridium monoculture as it was associated to glucose. The use of co-cultures with starchy substrates up to 150 g L−1 also exhibited an increment in total ABE production in a range of 37 to 116% over its monocultures (Table 4) [27, 29, 41]. These easier assimilable residues composed mainly by glucose led to increments in either the ABE yield [29] or the productivities [27, 41] (Table 4). In the sense of lignocellulosic substrates, it seems that the Clostridium species has a significant impact. Using C. beijerinckii the butanol production increased from 4.22 to 10.62 g L−1 by implementing the co-culture system with S. cerevisiae [42], which was not evident with C. acetobutylicum. Our results support that the co-culture strategy with Clostridium species and S. cerevisiae can extend ABE concentrations not only with starchy substrates, but also with lignocellulosic wastes. Furthermore, this co-culture strategy enhances the exploitation of the substrate.
4 Conclusions
The main aim of this work was to evaluate the integration of pH regulation and a co-culture system of C. acetobutylicum and S. cerevisiae with the target to improve xylose consumption and subsequent solvent concentrations from RS hydrolysate. The modulation of pH by using ammonium acetate as buffer and active minimum pH control at 4.8 was succesfully applied to RS hydrolysate. The use of the co-culture of C. acetobutylicum and S. cerevisiae led to an increase of 1.4-fold of the total solvent concentration, mainly due to ethanol production. Moreover, S. cerevisiae promoted the xylose uptake by C. acetobutylicum, probably associated due to the amino acids excreted by the yeast, thus enhancing the overall exploitation of the RS. The better exploitation of the secondary monosaccharaide of hydrolysates, xylose, in the co-culture was correlated to butanol production by Clostridium species. This co-culture fermentation strategy can be used to increment butanol and ethanol co-production and sugar consumption from lignocellulosic wastes.
References
de Jong E, Stichnothe H, Bell G, Jørgensen H (2020) Bio-based chemicals: a 2020 update — Task 42. IEA Bioenergy
Dürre P (2007) Biobutanol: an attractive biofuel. Biotechnol J 2:1525–1534. https://doi.org/10.1002/biot.200700168
Bharathiraja B, Jayamuthunagai J, Sudharsanaa T et al (2017) Biobutanol — an impending biofuel for future: a review on upstream and downstream processing tecniques. Renew Sustain Energy Rev 68:788–807
Ibrahim MF, Ramli N, Kamal Bahrin E, Abd-Aziz S (2017) Cellulosic biobutanol by Clostridia: challenges and improvements. Renew Sustain Energy Rev 79:1241–1254. https://doi.org/10.1016/j.rser.2017.05.184
Xue C, Zhao XQ, Liu CG et al (2013) Prospective and development of butanol as an advanced biofuel. Biotechnol Adv 31:1575–1584
Mood SH, Golfeshan AH, Tabatabaei M et al (2013) Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew Sustain Energy Rev 27:77–93
Birgen C, Dürre P, Preisig HA, Wentzel A (2019) Butanol production from lignocellulosic biomass: revisiting fermentation performance indicators with exploratory data analysis. Biotechnol Biofuels 12:167
Ren C, Gu Y, Hu S et al (2010) Identification and inactivation of pleiotropic regulator CcpA to eliminate glucose repression of xylose utilization in Clostridium acetobutylicum. Metab Eng 12:446–454. https://doi.org/10.1016/j.ymben.2010.05.002
Zhang J, Zhu W, Xu H et al (2016) Simultaneous glucose and xylose uptake by an acetone/butanol/ethanol producing laboratory Clostridium beijerinckii strain SE-2. Biotechnol Lett 38:611–617. https://doi.org/10.1007/s10529-015-2028-5
Xiao H, Li Z, Jiang Y et al (2012) Metabolic engineering of d-xylose pathway in Clostridium beijerinckii to optimize solvent production from xylose mother liquid. Metab Eng 14:569–578. https://doi.org/10.1016/j.ymben.2012.05.003
Gu Y, Li J, Zhang L et al (2009) Improvement of xylose utilization in Clostridium acetobutylicum via expression of the talA gene encoding transaldolase from Escherichia coli. J Biotechnol 143:284–287. https://doi.org/10.1016/j.jbiotec.2009.08.009
Liu L, Zhang L, Tang W et al (2012) Phosphoketolase pathway for xylose catabolism in Clostridium acetobutylicum revealed by 13C metabolic flux analysis. J Bacteriol 194:5413–5422. https://doi.org/10.1128/JB.00713-12
Liu J, Liu Z, Guo T (2018) Repeated-batch fermentation by immobilization of Clostridium beijerinckii NCIMB 8052 in a fibrous bed bioreactor for ABE (acetone-butanol-ethanol) production. J Renew Sustain Energy 10:013101. https://doi.org/10.1063/1.5007133
Zhang H, Yang P, Wang Z et al (2021) Clostridium acetobutylicum biofilm: advances in understanding the basis. Front Bioeng Biotechnol 9:459. https://doi.org/10.3389/fbioe.2021.658568
Capilla M, San-Valero P, Izquierdo M et al (2021) The combined effect on initial glucose concentration and pH control strategies for acetone-butanol-ethanol (ABE) fermentation by Clostridium acetobutylicum DSM 792. Biochem Eng J 167:107910. https://doi.org/10.1016/j.bej.2020.107910
Survase SA, Zebroski R, Bayuadri C et al (2019) Membrane assisted continuous production of solvents with integrated solvent removal using liquid-liquid extraction. Bioresour Technol 280:378–386. https://doi.org/10.1016/j.biortech.2019.02.024
Procentese A, Raganati F, Olivieri G et al (2015) Continuous xylose fermentation by Clostridium acetobutylicum — assessment of solventogenic kinetics. Bioresour Technol 192:142–148. https://doi.org/10.1016/j.biortech.2015.05.041
Procentese A, Raganati F, Olivieri G et al (2014) Continuous xylose fermentation by Clostridium acetobutylicum — kinetics and energetics issues under acidogenesis conditions. Bioresour Technol 164:155–161. https://doi.org/10.1016/j.biortech.2014.04.054
Raganati F, Olivieri G, Götz P et al (2015) Butanol production from hexoses and pentoses by fermentation of Clostridium acetobutylicum. Anaerobe 34:146–155. https://doi.org/10.1016/j.anaerobe.2015.05.008
El Kanouni A, Zerdani I, Zaafa S et al (1998) The improvement of glucose/xylose fermentation by Clostridium acetobutylicum using calcium carbonate. World J Microbiol Biotechnol 14:431–435. https://doi.org/10.1023/A:1008881731894
Jiang W, Wen Z, Wu M et al (2014) The effect of pH control on acetone-butanol-ethanol fermentation by Clostridium acetobutylicum ATCC 824 with xylose and D-glucose and D-xylose mixture. Chinese J Chem Eng 22:937–942. https://doi.org/10.1016/j.cjche.2014.06.003
Bader J, Mast-Gerlach E, Popović MK et al (2010) Relevance of microbial coculture fermentations in biotechnology. J Appl Microbiol 109:371–387
Shahab RL, Luterbacher JS, Brethauer S, Studer MH (2018) Consolidated bioprocessing of lignocellulosic biomass to lactic acid by a synthetic fungal-bacterial consortium. Biotechnol Bioeng 115:1207–1215. https://doi.org/10.1002/bit.26541
Lee WS, Chen IC, Chang CH, Yang SS (2012) Bioethanol production from sweet potato by co-immobilization of saccharolytic molds and Saccharomyces cerevisiae. Renew Energy 39:216–222. https://doi.org/10.1016/j.renene.2011.08.024
Verma G, Nigam P, Singh D, Chaudhary K (2000) Bioconversion of starch to ethanol in a single-step process by coculture of amylolytic yeasts and Saccharomyces cerevisiae 21. Bioresour Technol 72:261–266. https://doi.org/10.1016/S0960-8524(99)00117-0
Wu J, Dong L, Zhou C et al (2019) Developing a coculture for enhanced butanol production by Clostridium beijerinckii and Saccharomyces cerevisiae. Bioresour Technol Reports 6:223–228. https://doi.org/10.1016/j.biteb.2019.03.006
Luo H, Ge L, Zhang J et al (2015) Enhancing butanol production under the stress environments of co-culturing Clostridium acetobutylicum/Saccharomyces cerevisiae integrated with exogenous butyrate addition. PLoS ONE 10:e0141160. https://doi.org/10.1371/journal.pone.0141160
Luo H, Zeng Q, Han S et al (2017) High-efficient n-butanol production by co-culturing Clostridium acetobutylicum and Saccharomyces cerevisiae integrated with butyrate fermentative supernatant addition. World J Microbiol Biotechnol 33:76. https://doi.org/10.1007/s11274-017-2246-1
Qi G, Xiong L, Luo M et al (2018) Solvents production from cassava by co-culture of Clostridium acetobutylicum and Saccharomyces cerevisiae. J Environ Chem Eng 6:128–133. https://doi.org/10.1016/j.jece.2017.11.067
Valles A, Capilla M, Álvarez-Hornos FJ et al (2021) Optimization of alkali pretreatment to enhance rice straw conversion to butanol. Biomass Bioenerg 150:106131. https://doi.org/10.1016/j.biombioe.2021.106131
Qureshi N, Saha BC, Klasson KT, Liu S (2018) Butanol production from sweet sorghum bagasse with high solids content: part I—comparison of liquid hot water pretreatment with dilute sulfuric acid. Biotechnol Prog 34:960–966. https://doi.org/10.1002/btpr.2639
Folin O, Denis W (1912) On phosphotungstic-phosphomolybdic compounds as color reagents. J Biol Chem 12:239–243
Is M, Steiner E, Hirsch S et al (2000) The cause of “acid crash” and “acidogenic fermentations” during the batch acetone-butanol-ethanol (ABE-) fermentation process. J Mol Microbiol Biotechnol 2:95–100
Luo H, Zheng P, Xie F et al (2019) Co-production of solvents and organic acids in butanol fermentation by: Clostridium acetobutylicum in the presence of lignin-derived phenolics. RSC Adv 9:6919–6927. https://doi.org/10.1039/c9ra00325h
Jiang M, Chen JN, He AY et al (2014) Enhanced acetone/butanol/ethanol production by Clostridium beijerinckii IB4 using pH control strategy. Process Biochem 49:1238–1244. https://doi.org/10.1016/j.procbio.2014.04.017
Ounine K, Petitdemange H, Raval G, Gay R (1985) Regulation and butanol inhibition of D-xylose and D-glucose uptake in Clostridium acetobutylicum. Appl Environ Microbiol 49:874–878. https://doi.org/10.1128/aem.49.4.874-878.1985
Amiri H, Karimi K, Zilouei H (2014) Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour Technol 152:450–456. https://doi.org/10.1016/j.biortech.2013.11.038
Qi GX, Xiong L, Huang C et al (2015) Solvents production from a mixture of glucose and xylose by mixed fermentation of Clostridium acetobutylicum and Saccharomyces cerevisiae. Appl Biochem Biotechnol 177:996–1002. https://doi.org/10.1007/s12010-015-1790-0
Jin Q, Qureshi N, Wang H, Huang H (2019) Acetone-butanol-ethanol (ABE) fermentation of soluble and hydrolyzed sugars in apple pomace by Clostridium beijerinckii P260. Fuel 244:536–544. https://doi.org/10.1016/j.fuel.2019.01.177
Moradi F, Amiri H, Soleimanian-Zad S et al (2013) Improvement of acetone, butanol and ethanol production from rice straw by acid and alkaline pretreatments. Fuel 112:8–13. https://doi.org/10.1016/j.fuel.2013.05.011
Luo H, Zhang J, Wang H et al (2017) Effectively enhancing acetone concentration and acetone/butanol ratio in ABE fermentation by a glucose/acetate co-substrate system incorporating with glucose limitation and C. acetobutylicum/S. cerevisiae co-culturing. Biochem Eng J 118:132–142. https://doi.org/10.1016/j.bej.2016.12.003
Wu J, Dong L, Liu B, et al (2020) A novel integrated process to convert cellulose and hemicellulose in rice straw to biobutanol. Environ Res 186https://doi.org/10.1016/j.envres.2020.109580
Acknowledgements
We would like to thank Professor Emilia Matallana and Professor Agustin Aranda (I2SysBio UV-CSIC) for their gift of S. cerevisiae EYS4.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Grant CTM2017-88042-R funded by MCIN/AEI/ https://doi.org/10.13039/501100011033 and by “ERDF: a way of making Europe.” This research also received funding from the Conselleria d’Innovacio, Universitats, Ciencia i Societat Digital, Generalitat Valenciana, Spain (AICO/2021/121). Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. M. Capilla and A. Valles acknowledges Generalitat Valenciana (Spain) and the European Social Fund for their contracts (ACIF/2019/138 and ACIF/2017/390). The funding sources had no involvement in this work.
Author information
Authors and Affiliations
Contributions
M. Capilla: investigation, data curation, formal analysis, writing — original draft, visualization. A. Valles: conceptualization, methodology, visualization. P. San-Valero: conceptualization, methodology, investigation, writing — review and editing, supervision. F. J. Álvarez-Hornos: conceptualization, methodology, resources. C. Gabaldón: conceptualization, methodology, investigation, writing — review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Capilla, M., Valles, A., San-Valero, P. et al. Solvent production from rice straw by a co-culture of Clostridium acetobutylicum and Saccharomyces cerevisiae: effect of pH control. Biomass Conv. Bioref. 14, 5561–5573 (2024). https://doi.org/10.1007/s13399-022-02750-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02750-4