Abstract
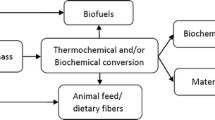
Pomegranate peel (PP) constitutes 40–50 % of the total weight of the pomegranate fruit, thereby accounting for the peel waste nine times as compared to the quantity of fruit processed, thus posing the challenge of solid waste management. Sustainable management of PP through circular bioeconomy can be done by applying resource life-extending strategies. The present work uses this concept and reports valorization of PP with zero waste using the biorefinery approach. High-value products like polyphenol and dietary fibers were recovered sequentially from PP using greener deep eutectic solvents (DESs). PPs were initially treated with eleven adaptable DESs for the extraction of polyphenols using choline chloride (ChCl) as a hydrogen bond acceptor (HBA) and ethylene glycol (EG), glycerol (Gly), malic acid (MA), and urea (U) as hydrogen bond donors (HBDs). Furthermore, based on the total phenolic contents (TPCS), four DESs with the best (ChCl:EG (1:2) and ChCl:Gly (1:3)), intermediate (ChCl:MA (1:1)), and the least (ChCl:U (1:2)) efficiency were selected for characterization (FTIR and viscosity) and detailed evaluation for maximum extraction of TPC. The obtained extracts were subjected to qualitative/quantitative test (LC-MS/MS) to assess the presence of phytochemicals/six phenolic acids along with the antioxidant (ferric-reducing antioxidant power/ABTS assay) and antibacterial (Escherichia coli and Staphylococcus aureus) ability. The PP and its residues were subjected to SEM and CHNS analysis and depicted structural variations proving DES to be an effective solvent for dissolving the cell wall. The residue obtained after extraction was analyzed for the total dietary fibers (51.08–41.92 %) to ensure the complete usage of the PP with zero waste.








Similar content being viewed by others
References
de Brito Nogueira TB, da Silva TPM, de Araújo Luiz D et al (2020) Fruits and vegetable-processing waste: a case study in two markets at Rio de Janeiro, RJ, Brazil. Environ Sci Pollut Res 27:18530–18540. https://doi.org/10.1007/s11356-020-08244-y
Dey T, Bhattacharjee T, Nag P et al (2021) Valorization of agro-waste into value added products for sustainable development. Bioresour Technol Rep 16:100834. https://doi.org/10.1016/j.biteb.2021.100834
Ozturk B, Parkinson C, Gonzalez-Miquel M (2018) Extraction of polyphenolic antioxidants from orange peel waste using deep eutectic solvents. Sep Purif Technol 206:1–13. https://doi.org/10.1016/j.seppur.2018.05.052
Hernández-Corroto E, Plaza M, Marina ML, García MC (2020) Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innov Food Sci Emerg Technol 60:102314. https://doi.org/10.1016/J.IFSET.2020.102314
Suleria HAR, Barrow CJ, Dunshea FR (2020) Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 9https://doi.org/10.3390/foods9091206
Brahem M, Renard CMGC, Eder S et al (2017) Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res Int 95:125–133. https://doi.org/10.1016/j.foodres.2017.03.002
Kyriakidou A, Makris DP, Lazaridou A, Biliaderis CG, Mourtzinos I (2021) Physical properties of chitosan films containing pomegranate peel extracts obtained by deep eutectic solvents. Foods 10(6):1262. https://doi.org/10.3390/foods10061262
Rajha HN, Mhanna T, El Kantar S et al (2019) Innovative process of polyphenol recovery from pomegranate peels by combining green deep eutectic solvents and a new infrared technology. LWT 111:138–146. https://doi.org/10.1016/J.LWT.2019.05.004
Tito A, Colantuono A, Pirone L et al (2021) Pomegranate peel extract as an inhibitor of SARS-CoV-2 spike binding to human ACE2 Receptor (in vitro): a promising source of novel antiviral drugs. Front Chem 9:1–37. https://doi.org/10.3389/fchem.2021.638187
Shinde PN, Mandavgane SA, Karadbhajane V (2020) Process development and life cycle assessment of pomegranate biorefinery. Environ Sci Pollut Res 27:25785–25793. https://doi.org/10.1007/s11356-020-08957-0
Talekar S, Patti AF, Vijayraghavan R, Arora A (2018) An integrated green biorefinery approach towards simultaneous recovery of pectin and polyphenols coupled with bioethanol production from waste pomegranate peels. Bioresour Technol 266:322–334. https://doi.org/10.1016/j.biortech.2018.06.072
Khan S, Patel A, Bhise KS (2017) Antioxidant activity of pomegranate peel powder. J Drug Deliv Ther 7:992–997 https://doi.org/10.22270/jddt.v7i2.1380
Hasnaoui N, Wathelet B, Jiménez-Araujo A (2014) Valorization of pomegranate peel from 12 cultivars: dietary fibre composition, antioxidant capacity and functional properties. Food Chem 160:196–203. https://doi.org/10.1016/j.foodchem.2014.03.089
Wu L, Li L, Chen S, et al (2020) Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: optimization, comparison and antioxidant activity. Sep Purif Technol 247:117014 https://doi.org/10.1016/J.SEPPUR.2020.117014
Wojeicchowski JP, Marques C, Igarashi-Mafra L, et al (2021) Extraction of phenolic compounds from rosemary using choline chloride – based deep eutectic solvents. Sep Purif Technol 258https://doi.org/10.1016/j.seppur.2020.117975
Cai C, Wu S, Wang C et al (2019) Deep eutectic solvents used as adjuvants for improving the salting-out extraction of ursolic acid from Cynomorium songaricum Rupr. in aqueous two-phase system. Sep Purif Technol 209:112–118. https://doi.org/10.1016/j.seppur.2018.07.017
Benvenutti L, Sanchez-Camargo A del P, Zielinski AAF, Ferreira SRS (2020) NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: in silico and experimental approaches for solvent selection. J Mol Liq 315:113761 https://doi.org/10.1016/J.MOLLIQ.2020.113761
Fanali C, Gallo V, Posta S Della, et al (2021) Choline chloride–lactic acid-based NADES as an extraction medium in a response surface methodology-optimized method for the extraction of phenolic compounds from hazelnut skin. Molecules 26https://doi.org/10.3390/molecules26092652
Peng X, Duan MH, Yao XH et al (2016) Green extraction of five target phenolic acids from Lonicerae japonicae Flos with deep eutectic solvent. Sep Purif Technol 157:249–257. https://doi.org/10.1016/J.SEPPUR.2015.10.065
Korekar G, Stobdan T, Singh H et al (2011) Phenolic content and antioxidant capacity of various solvent extracts from seabuckthorn (Hippophae rhamnoides L.) fruit pulp, seeds, leaves and stem bark. Acta Aliment 40:449–458. https://doi.org/10.1556/AALIM.40.2011.4.4
Chan EWC, Lim YY, Chew YL (2007) Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem 102:1214–1222. https://doi.org/10.1016/J.FOODCHEM.2006.07.009
Kumar N, Upadhyay P, Saxena G (2018) Phytochemical screening of pomegranate peel using crude hydro-alcoholic extract and pharmacological activities. J Sci Res Publications 8(1):193–199
Karthikeyan G, Vidya AK (2019) Phytochemical analysis, antioxidant and antibacterial activity of pomegranate peel. Life Sci Informatics Publ 5:218–231 https://doi.org/10.26479/2019.0501.22
CBT P, GC J (2019) Deep eutectic solvent-based extraction of polyphenolic antioxidants from onion (Allium cepa L.) peel. J Sci Food Agric 99:1969–1979https://doi.org/10.1002/JSFA.9395
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76. https://doi.org/10.1006/abio.1996.0292
Re R, Pellegrini N, Proteggente A et al (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Benzie IFF, Devaki M (2017) The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: concepts, procedures, limitations and applications. Meas Antioxid Act Capacit Recent Trends Appl 77–106https://doi.org/10.1002/9781119135388.CH5
Silva JM, Silva E, Reis RL, Duarte ARC (2019) A closer look in the antimicrobial properties of deep eutectic solvents based on fatty acids. Sustain Chem Pharm 14:100192. https://doi.org/10.1016/j.scp.2019.100192
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal 6(2):71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Asp NG, Johansson CG, Hallmer H, Siljestróm M (1983) Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agric Food Chem 31:476–482. https://doi.org/10.1021/jf00117a003
Choi YH, Verpoorte R (2019) Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr Opin Food Sci 26:87–93
Khanpit VV, Tajane SP, Mandavgane SA (2021) Dietary fibers from fruit and vegetable waste: methods of extraction and processes of value addition. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01980-2
Kupnik K, Leitgeb M, Primožič M, Postružnik V, Kotnik P, Kučuk N, Knez Ž, Marevci MK (2022) Supercritical fluid and conventional extractions of high value-added compounds from pomegranate peels waste: Production, quantification and antimicrobial activity of bioactive constituents. Plants 11(7):928. https://doi.org/10.3390/plants11070928
Hayyan M, Abo-Hamad A, AlSaadi MAH, Hashim MA (2015) Functionalization of graphene using deep eutectic solvents. Nanoscale Res Lett 10https://doi.org/10.1186/s11671-015-1004-2
Crossey LJ (1991) Thermal degradation of aqueous oxalate species. Geochim Cosmochim Acta 55:1515–1527. https://doi.org/10.1016/0016-7037(91)90124-N
Delgado-Mellado N, Larriba M, Navarro P et al (2018) Thermal stability of choline chloride deep eutectic solvents by TGA/FTIR-ATR analysis. J Mol Liq 260:37–43. https://doi.org/10.1016/j.molliq.2018.03.076
Wang Y, Ma C, Liu C et al (2020) Thermodynamic study of choline chloride-based deep eutectic solvents with water and methanol. J Chem Eng Data 65:2446–2457. https://doi.org/10.1021/acs.jced.9b01113
Abbott AP, Harris RC, Ryder KS et al (2011) Glycerol eutectics as sustainable solvent systems. Green Chem 13:82–90. https://doi.org/10.1039/c0gc00395f
Pastoriza-Gallego MJ, Lugo L, Legido JL, Piñeiro MM (2011) Thermal conductivity and viscosity measurements of ethylene glycol-based Al2O3 nanofluids. Nanoscale Res Lett 6(1):1–11. https://doi.org/10.1186/1556-276X-6-221
Zhang Q, De Oliveira Vigier K, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41:7108–7146. https://doi.org/10.1039/c2cs35178a
Agieienko V, Buchner R (2021) A comprehensive study of density, viscosity, and electrical conductivity of (choline chloride + glycerol) deep eutectic solvent and its mixtures with dimethyl sulfoxide. J Chem Eng Data 66:780–792. https://doi.org/10.1021/acs.jced.0c00869
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114:11060–11082. https://doi.org/10.1021/CR300162P
Spigno G, Tramelli L, De Faveri DM (2007) Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng 81:200–208. https://doi.org/10.1016/J.JFOODENG.2006.10.021
Wang H, Ma X, Cheng Q, Wang L, Zhang L (2018) Deep eutectic solvent-based ultrahigh pressure extraction of baicalin from Scutellaria baicalensis Georgi. Molecules 23(12):3233. https://doi.org/10.3390/molecules23123233
Mota FL, Queimada AJ, Pinho SP, Macedo EA (2008) Aqueous solubility of some natural phenolic compounds. Ind Eng Chem Res 47:5182–5189. https://doi.org/10.1021/IE071452O
Durling NE, Catchpole OJ, Grey JB et al (2007) Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol–water mixtures. Food Chem 101:1417–1424. https://doi.org/10.1016/J.FOODCHEM.2006.03.050
Brahmi F, Merchiche F, Mokhtari S, et al (2021) Optimization of some extraction parameters of phenolic content from apple peels and grape seeds and enrichment of yogurt by their powders: a comparative study. J Food Process Preserv 45https://doi.org/10.1111/JFPP.15126
Nam MW, Zhao J, Lee MS et al (2015) Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from Flos sophorae. Green Chem 17:1718–1727. https://doi.org/10.1039/c4gc01556h
Friedman M, Kozukue N, Kim HJ et al (2017) Glycoalkaloid, phenolic, and flavonoid content and antioxidative activities of conventional nonorganic and organic potato peel powders from commercial gold, red, and Russet potatoes. J Food Compos Anal 62:69–75. https://doi.org/10.1016/j.jfca.2017.04.019
Yağmur N, Şahin S (2020) Encapsulation of ellagic acid from pomegranate peels in microalgae optimized by response surface methodology and an investigation of its controlled released under simulated gastrointestinal studies. J Food Sci 85:998–1006. https://doi.org/10.1111/1750-3841.15085
Sepúlveda L, Ascacio A, Rodríguez-Herrera R et al (2011) Ellagic acid: biological properties and biotechnological development for production processes. Afr J Biotechnol 10:4518–4523. https://doi.org/10.1002/chin.201250260
Kilic I, Yeşiloǧlu Y, Bayrak Y (2014) Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim Acta - Part A Mol Biomol Spectrosc 130:447–452. https://doi.org/10.1016/j.saa.2014.04.052
Wen Q, Chen JX, Tang YL et al (2015) Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 132:63–69. https://doi.org/10.1016/j.chemosphere.2015.02.061
Sousa JM, de Souza EL, Marques G et al (2016) Polyphenolic profile and antioxidant and antibacterial activities of monofloral honeys produced by Meliponini in the Brazilian semiarid region. Food Res Int 84:61–68. https://doi.org/10.1016/j.foodres.2016.03.012
Chen Y, Mu T (2019) Application of deep eutectic solvents in biomass pretreatment and conversion. Green Energy Environ 4:95–115. https://doi.org/10.1016/j.gee.2019.01.012
Lu W, Liu S (2020) Choline chloride–based deep eutectic solvents (Ch-DESs) as promising green solvents for phenolic compounds extraction from bioresources: state-of-the-art, prospects, and challenges. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00753-7
Acknowledgements
The authors express their gratitude to the anonymous reviewers and the editor in chief, Martin Kaltschmitt, for their efforts, time, and recommendations.
Funding
The Ministry of Food Processing Industries (MoFPI), Govt. of India, provided financial support (sanction number Q-11/18/2019-R&D) to Dr. Anupama Kumar to carry out the above work.
Author information
Authors and Affiliations
Contributions
Shivali Singh Gaharwar: investigation, methodology, analysis, validation, writing—original draft preparation. Anupama Kumar: supervision, conceptualization, visualization, funding acquisition, project administration, reviewing and editing. Sachin A. Mandavgane: reviewing and editing. Rashmi Rahagude: data curation, formal analysis. Shital Gokhale: formal analysis, data curation. K. Yadav: formal analysis. A. P. Barua: formal analysis.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gaharwar, S.S., Kumar, A., Mandavgane, S.A. et al. Valorization of Punica granatum (pomegranate) peels: a case study of circular bioeconomy. Biomass Conv. Bioref. 14, 7707–7724 (2024). https://doi.org/10.1007/s13399-022-02744-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02744-2