Abstract
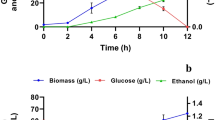
In this study, bioethanol production was compared via a novel two-stage fermentation using Zymomonas mobilis and/or Pichia stipitis with low inoculum size (5%). A synthetic glucose (30 g/l)/xylose (20 g/l) medium simulated the real hydrolyzed lignocellulose, and the inhibitory effects of different compounds and high sugar and bioethanol concentrations were restricted to find strains synergistic interactions. Higher ethanol yield and sugar conversion alongside lower time and cost were also considered. Xylose was entirely consumed by single P. stipitis only, but glucose was completely fermented in all strategies. Maximum glucose and xylose conversion efficiencies were obtained by single Z. mobilis and P. stipitis cultures, respectively. Sequential culture provided the same glucose conversion and consumption rate (100% to 1.01 gg/l.h) and yield (0.50 ge/gg) as single Z. mobilis and the nearest xylose conversion efficiency (30% to 0.14 gx/l.h to 0.16 ge/gx) to single P. stipitis (47% to 0.34 gx/l.h to 0.27 ge/gx). However, this culture was inefficient since Z. mobilis was removed. By individually immobilizing the strains on stable calcium alginate beads, the sequential-co-immobilized strategy resulted in higher xylose conversion, yield, and overall productivity of 83%, 0.45 ge/gx, and 0.30 ge/l.h than the sequential-co culture (16%, 0.31 ge/gx, and 0.14 ge/l.h), respectively. This strategy provides synergistic interactions by allocating a part of glucose to rapid growth of P. stipitis and utilizing intermediate metabolites of xylose fermentation by Z. mobilis to enhance overall efficiency. The sequential-co-immobilized strategy was also examined in 2 L bioreactor where xylose uptake rate, xylose conversion, and ethanol productivity increased 0.04 gx/l.h, 11%, and 0.27 ge/l.h compared to the sequential-co, respectively. The sequential-co-immobilized culture was found as an efficient strategy in production of second-generation bioethanol from lignocellulose.
Graphical abstract







Similar content being viewed by others
References
Safaripour M, Ghanbari A, Seyedabadi E (2021) Investigation of environmental impacts of bioethanol production from wheat straw in Kermanshah. Iran Biomass Conv Bioref 31:11
Domínguez E, Nóvoa T, Del Río P, Garrote G, Romaní A (2020) Sequential two-stage autohydrolysis biorefinery for the production of bioethanol from fast-growing Paulownia biomass. Energy Convers. Manag. 226:113517
Casabar T, Unpaprom Y, Ramaraj R (2019) Fermentation of pineapple fruit peel wastes for bioethanol production. Biomass Conv Bioref 9:761–765
Lu J, Song F, Liu H, Chang C, Cheng Y, Wang H (2021) Production of high concentration bioethanol from reed by combined liquid hot water and sodium carbonate-oxygen pretreatment. Energy. 217:119332
Priyadharsini P, Dawn S (2021) Optimization of fermentation conditions using response surface methodology (RSM) with kinetic studies for the production of bioethanol from rejects of Kappaphycus alvarezii and solid food waste. Biomass Conv. Bioref.
Cui J, Hu P, Li Y (2021) Effects of non-ionic surfactants on bioethanol production using extracted cellulose from wheat straw and kinetic studies. Biomass Conv. Bioref.
Fu N, Peiris P, Markham J, Bavor J (2009) A novel co-culture process with Zymomonas mobilis and Pichia stipitis for efficient ethanol production on glucose/xylose mixtures. Enzyme Microb Technol 45:210–217
Ben Atitallah I, Arous F, Louati I, Zouari-Mechichi H, Brysch-Herzberg M, Woodward S, Mechichi T (2021) Efficient bioethanol production from date palm (Phoenix dactylifera L.) sap by a newly isolated Saccharomyces cerevisiae X19G2. Process Biochem 105:102–112
Verma N, Kumar V (2021) Microbial conversion of waste biomass into bioethanol: current challenges and future prospects. Biomass Conv Bioref.
Tahir B, Mezori A (2020) Bioethanol production from Quercus aegilops using Pichia stipitis and Kluyveromyces marxianus. Biomass Conv Bioref
Singh L, Majumder C, Ghosh S (2014) Development of sequential-co-culture system (Pichia stipitis and Zymomonas mobilis) for bioethanol production from Kans grass biomass. Biochem Eng J 82:150–157
Mustafa I, Elkamel A, Lohi A, Ibrahim G, Elnashaie S (2014) Structured mathematical modeling, bifurcation, and simulation for the bioethanol fermentation process using Zymomonas mobilis. Ind Eng Chem Res 53:5954–5972
Chen Y (2011) Development and application of co-culture for ethanol production byco-fermentation of glucose and xylose: a systematic review. J Ind Microbiol Biotechnol 38:581–597
Silva J, Mussatto S, Roberto I, Teixeira J (2012) Fermentation medium andoxygen transfer conditions that maximize the xylose conversion to ethanol by Pichia stipitis. Renew Energy 37:259–265
Isabella D, Paola D, Daniela C, Federico C (2013) Angela C and Patrizia R, Bioethanol production from mixed sugars by Scheffersomyces stipitis free and immobilized cells, and co-cultures with Saccharomyces cerevisiae. N Biotechnol 30:1–7
Jeffries T (2006) Engineering yeasts for xylose metabolism. Curr Opin Biotechnol 17:320–326
Lin Y, Tanaka S (2006) Ethanol fermentation from biomass resources: current state and prospects. Appl Microbiol Biotechnol 69:627–642
Karagöz P, Özkan M (2014) Ethanol production from wheat straw by Saccharomyces cerevisiae and Scheffersomyces stipitis co-culture in batch and continuous system. Bioresour Technol 158:286–293
Thi D, Nguyen T, Praveen P, Loh K (2019) Co-culture of Zymomonas mobilis and Scheffersomyces stipitis immobilized in polymeric membranes for fermentation of glucose and xylose to ethanol. Biochem Eng J 145:145–152
Flevaris K, Chatzidoukas C (2021) Optimal fed-batch bioreactor operating strategies for the microbial production of lignocellulosic bioethanol and exploration of their economic implications: a step forward towards sustainability and commercialization. J. Clean. Prod. 295:126384
Malik K, Salama E (2021) El-Dalatony M, Jalalah M, Harraz F, Al-Assiri M, Zheng Y, Sharma P and Li X, Co-fermentation of immobilized yeasts boosted bioethanol production from pretreated cotton stalk lignocellulosic biomass: long-term investigation. Ind. Crops Prod. 159:113122
Mathew A, Crook M, Chaney K, Humphries A (2013) Comparison of entrapment and biofilm mode of immobilisation for bioethanol production from oilseed rape straw using Saccharomyces cerevisiae cells. Biom Bioen 52:1–7
Günan Yücel H, Aksu Z (2015) Ethanol fermentation characteristics of Pichia stipitis yeast from sugar beet pulp hydrolysate: use of new detoxification methods. Fuel 158:793–799
Cai D, Hu S, Chen C, Wang Y, Zhang C, Miao Q, Qin P, Tan T (2016) Immobilized ethanol fermentation coupled to pervaporation with silicalite-1/polydimethylsiloxane/polyvinylidene fluoride composite membrane. Bioresour Technol 220:124
Li J, Zhou W, Fan S, Xiao Z, Liu Y, Liu J, Qiu B, Wang Y (2018) Bioethanol production in vacuum membrane distillation bioreactor by permeate fractional condensation and mechanical vapor compression with polytetrafluoroethylene (PTFE) membrane. Bioresour Technol 268:708
Zhao J, Xu Y, Wang W, Griffin J, Roozeboom K, Wang D (2020) Bioconversion of industrial hemp biomass for bioethanol production: a review. Fuel. 281:118725
Chen Y (2011) Development and application of co-culture for ethanol production by co-fermentation of glucose and xylose: a systematic review. J Ind Microbiol Biotechnol 38:581
Da Cunha-Pereira F, Hickert L, Sehnem N, De Souza-Cruz P, Rosa C, Ayub Z (2011) Conversion of sugars present in rice hull hydrolysates into ethanol by Spathaspora arborariae, Saccharomyces cerevisiae, and their co-fermentations. Biores Technol 102(5):4218
Sieuwerts S, De Bok F, Mols E, De Vos W, Van Hylckama J (2008) A simple and fast method for determining colony forming units. Lett Appl Microbiol 47:275–278
Medina C, Estrada C, Hernandez D, Jesus C, Torres A, Saldivar R (2017) Current state of bioethanol fuel blends in Mexico. Biofues Bioproduc Bioref 12:338–347
Fernell W, King H (1953) The simultaneous determination of pentose and hexose in mixtures of sugars. Analyst 78:80–83
Caputi A, Ueda M, Brown T (1968) Spectrophotometric determination of ethanol in wine. Am J Enol Vitic 19:160–165
Chen L, Chen R, Fu S (2015) FeCl3 pretreatment of three lignocellulosic biomass for ethanol production. ACS Sustain Chem Eng 3:1794–1800
Varjani S, Joshi R, Srivastava V, Ngo H, Guo W (2020) Treatment of wastewater from petroleum industry: current practices and perspectives. Environ Sci Pollut Res 27:27172–27180
Liu C, Xia J, Gu J, Wang W, Liu Q, Yan L, Chen T (2021) Multifunctional CNTs-PAA/MIL101(Fe)@Pt composite membrane for high-throughput oily wastewater remediation. J. Hazard. Mater. 403:123547
Toor M, Kumar S, Malyan S, Bishnoi N, Mathimani T, Rajendran K, Pugazhendhi A (2020) An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere. 242:125080
Bellido C, González-Benito G, Coca M, Lucas T, García-Cubero M (2013) Influence of aeration on bioethanol production from ozonized wheat straw hydrolysates using Pichia stipitis. Bioresour Technol 133:51–58
Wirawan F, Cheng C, Kao W, Lee D, Chang J (2012) Cellulosic ethanol production performance with SSF and SHF processes using immobilized Zymomonas mobilis. Appl Energy 100:19–26
Busiswa N, Chiyanzu I, Marx S, Obiero G (2014) Effect of Saccharomyces cerevisiae and Zymomonas mobilis on the co-fermentation of sweet sorghum bagasse hydrolysates pretreated under varying conditions. Biomass and Bioenergy 71:350–356
Lin T, Guo G, Hwang W, Huang S (2016) The addition of hydrolyzed rice straw in xylose fermentation by Pichia stipitis to increase bioethanol production at the pilot-scale. Biom Bioen 91:204–209
Nurhayati C, Cheng D, Nagarajan J, Chang (2016) Immobilization of Zymomonas mobilis with Fe2O3-modified polyvinyl alcohol for continuous ethanol fermentation. Biochem Eng J 114:298–306
Montalbano G, Toumpaniari S, Popov A, Duan P, Chen J, Dalgarno K, Scott W, Ferreira A (2018) Synthesis of bioinspired collagen/alginate/fibrin based hydrogels for soft tissue engineering. Mater Sci Eng C 91:236–246
Grootjen D, Meijlink L, Vleesenbeek R, Van der Lans R, Luyben K (1991) Cofermentation of glucose and xylose with immobilized Pichia stipitis in combination with Saccharomyces cerevisiae, Enzyme Microb. Technol 13:530–536
Beigbede J, de Medeiros DJ, Lavoie J (2021) Optimization of yeast, sugar and nutrient concentrations for high ethanol production rate using industrial sugar beet molasses and response surface methodology. Fermentation 7(2):86
Jafari O, Azin M, Moazami N (2022) Application of a statistical design to evaluate bioethanol production from Chlorella S4 biomass after acid -thermal pretreatment. Renew Energy 182:60
Dave N, Varadavenkatesan T, Selvaraj R, Vinayagam R (2021) Modelling of fermentative bioethanol production from indigenous Ulva prolifera biomass by Saccharomyces cerevisiae NFCCI1248 using an integrated ANN-GA approach. Sci. Total Environ. 791:148429
Sharma S, Rangaiah G (2012) Multi-objective optimization of a fermentation process integrated with cell recycling and inter-stage extraction. Comput Aided Chem Eng 31:860
Hussain A, Kangwa M, Yumnam N, Fernandez-Lahore M (2015) Operational parameters and their influence on particle-side mass transfer resistance in a packed bed bioreactor. AMB Express 5:51
Wang J, Chae M, Sauvageau D, Bressler D (2017) Improving ethanol productivity through self-cycling fermentation of yeast: a proof of concept. Biotechnol Biofuels 10:1–11
Patle S, Lal B (2008) Investigation of the potential of agro-industrial material as low cost substrate for ethanol production by using Candida tropicalis and Zymomonas mobilis. Biom Bioen 32:596–602
Fu N and Peiris A (2008) Co-fermentation of a mixture of glucose and xylose to ethanol by Zymomonas mobilis and Pachysolen tannophilus. 24: 1091–1097
Kamelian F, Mohammadi T, Naeimpoor F (2019) Fast, facile and scalable fabrication of novel microporous silicalite-1/PDMS mixed matrix membranes for efficient ethanol separation by pervaporation. Sep. Purif. Technol. 229:115820
Kamelian F, Mohammadi T, Naeimpoor F, Sillanpaä M (2020) One-step and low-cost designing of two-layered active-layer superhydrophobic silicalite-1/PDMS membrane for simultaneously achieving superior bioethanol pervaporation and fouling/biofouling resistance, ACS Appl. Mater Interfaces 12:56587–56603
Zhao Y, Yang X, Yan L, Bai Y, Li S, Sorokin P, Shao P (2021) Biomimetic nanoparticle-engineered superwettable membranes for efficient oil/water separation. J. Memb. Sci. 618:118525
Funding
Prof. Toraj Mohammadi would like to thank Iran National Science Foundation (INSF) for supporting the research (Grant number: 96008182).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
- Challenges of second-generation bioethanol were tested by fermenting glucose-xylose.
- Presence/absence strategies of Z. mobilis/P. stipitis were compared by two-stage process.
- Presence of Z. mobilis had adverse effect on xylose utilization in co-suspension.
- Removal of Z. mobilis from suspended culture increased operational time and cost.
- Sequential-co-immobilized culture improved xylose conversion from 16 to 83%.
- Scaling up fermentation increased xylose fermentation and overall performance.
Rights and permissions
About this article
Cite this article
Kamelian, F.S., Naeimpoor, F. & Mohammadi, T. Effect of Zymomonas mobilis and Pichia stipitis presence/absence strategies in a two-stage process on bioethanol production from glucose-xylose mixture. Biomass Conv. Bioref. 14, 3409–3424 (2024). https://doi.org/10.1007/s13399-022-02567-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02567-1