Abstract
The present study aimed to evaluate the sustainable utilization of date palm leaves (DPL) and rice straw (RS) as feed materials for ruminants using an in vitro wireless gas production (GP) technique. Date palm leaves and RS were individually ensiled with lactic or malic acids at 5 g/kg DM for 45 days. In a total mixed ration containing concentrate feed mixture, vegetable/fruits byproducts, untreated RS, and berseem hay (control ration), untreated RS was completely replaced with treated RS (ensiled without additives or with lactic or malic acids) whereas berseem hay was replaced with DPL (ensiled without additives or with lactic or malic acid) at 25, 50, 75, and 100% levels. Significant treatment × replacement level interactions were observed (P < 0.01) for most measured parameters of gas, methane (CH4) and carbon dioxide (CO2) productions, and degradability and volatile fatty acid (VFA) concentration. Replacing berseem hay with increasing levels of lactic or malic acid-treated DPL gradually decreased (P < 0.01) the asymptotic total gas and CH4 and CO2 productions, and malic acid-treated DPL decreased the rates of total gas, CH4 and CO2 productions, and the lag time of total GP. Moreover, lactic acid-treated DPL linearly increased (P < 0.05) the concentration of total VFA and acetate. Malic acid-treated DPL did not affect the measured ruminal fermentation parameters. Compared with the malate-treated DPL, lactate-treated DPL increased dry matter and neutral detergent fiber degradability, total VFA and acetate concentrations, and decreased CH4 production. In conclusion, replacing berseem hay with malic or lactic acids improved ruminal fermentation and decreased CH4 and CO2 productions, which can be considered as a sustainable strategy for cleaner ruminant production. Lactic acid treatment of DPL may result in better ruminal fermentation characteristics than the malic acid treatment of DPL.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Globally, large amounts of agriculture byproducts are produced annually, without a significant utilization [1]. Date palm (Phoenix dactilifera) leaves (DPL) are an example of the agricultural byproducts with a considerable potential nutritive value, but unsatisfactory use [2]. The nutritive value of DPL is hindered due to high concentration of fiber [3]. With about 20 kg of leaves produced from each tree, 14 million date palm trees generate approximately 650,000 tons of dry matter (DM) annually in Egypt [4]. The concentration of crude protein (CP) ranges from 5 to 16.5% while neutral detergent fiber (NDF) content varies from 42.7 to 72.4% in DPL [5, 6].
For many years, organic acids such as lactic acid, fumaric acid, malic acid, and formic acid have been used to improve the efficiency of beneficial ruminal microorganisms and fermentation [7, 8]. They are described as “Generally Recognized As Safe” and have been approved by the European Union for animal feeding [9]. Organic acids can stimulate the growth of ruminal Selenomonas ruminantium and increase the rate of protein hydrolysis [9, 10]. Moreover, they have been documented to stimulate ruminal bacterial growth and activity and enhance ruminant performance [8, 11]. Organic acids, such as lactic acid and malic acid, have been documented to reduce ruminal lactate production and stimulate ruminal lactate utilizing bacteria resulting in higher ruminal pH [12, 13]. Feeding organic acids increases the concentration of ruminal volatile fatty acids (VFAs) and propionate, and reduces methane (CH4) production by sinking hydrogen in the rumen [9, 13]. Elghangour et al. [7] evaluated different levels of organic acid salts (mainly calcium propionate and malate) on in vitro fermentation of diets with different silage to concentrate ratios, and observed increased gas production (GP) and reduced CH4 mitigation. Moreover, El-Zaiat et al. [8] observed that malic acid decreased in vitro CH4 production by 33%, and nutrient intake, but increased nutrient digestibility and milk production in lactating Holstein dairy cows. The effect of organic acids such as fumarate on CH4 mitigation was diet-dependent [13].
Organic acids such as acetate, propionate, or fumarate are applied to improve silage fermentation characteristics [14, 15]. Organic acids can decrease pH by direct acidification and retard the growth of undesirable spoilage bacteria such as aerobes and enterobacteria, thus ensuring favorable environment for rapid growth of lactic acid producing bacteria in silages [16]. They can directly inhibit the growth of molds and yeasts and improve the aerobic stability of silages [14, 16]. However, the effect of malic acid or lactic acid treatment to DPL on nutritional values of DPL is lacking. Therefore, effective treatments of low-quality forages and materials can be used as a method of improving their nutritive value and chemical composition. Jiang et al. [17] ensiled whole corn plant with organic acids and observed reduced NDF content in the silage. We hypothesized that malic acid or lactic acid treatment of DPL and RS during ensiling will raise the acidity rapidly, decrease the aerobic phase of fermentation, and accelerate the initiation of lag and anaerobic phases of silage, which may enhance the nutritional value of DPL and RS silage. Consequently, their use in the complete diets may reduce CH4 production and improve ruminal fermentation. Therefore, the objectives of the present experiment were to: (1) evaluate the nutritive value of DPL ensiled without additives or treated with lactic acid or malic acid and (2) assess different levels of DPL inclusion in the diets, replacing berseem hay on in vitro biogas (total gas, CH4 and carbon dioxide (CO2) productions) and in vitro ruminal fermentation.
2 Materials and methods
2.1 Ingredients and treatments
Fresh DPL were collected from different sites in the New Valley Governorate (Egypt) and sun-dried for 10 days [2]. Sun-dried rice straw (RS) was collected from local suppliers in Egypt. Date palm leaves, RS, and vegetable/fruits byproducts were individually ensiled under anaerobic conditions for 45 days using tightly closed plastic bags [18]. For DPL and RS, three types of silage were prepared (50 bags for DPL and 40 bags for RS), i.e., DPL and RS were ensiled without additives, with lactic acid or malic acid (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) at 5 g/kg DM. Before ensiling of dried chopped DPL (89.3% DM) and RS (94.3% DM), urea and molasses were included each at 4% (DM basis) to silages without additives, or silages treated with lactic acid or malic acid.
A basal total mixed ration (TMR) was prepared to be used as substrates, and contained (per kg DM) 500 g concentrate feed mixture, 100 g vegetable/fruits byproducts, 100 g untreated RS, and 300 g of berseem hay. Berseem hay was replaced with DPL (ensiled without additives or ensiled with lactic acid or malic acid) at 25, 50, 75, and 100%. Untreated RS in the control TMR was completely replaced with treated RS for lactic acid or malic acid-treated RS. Nutrient contents of ingredients and TMR are shown in Tables 1 and 2, respectively.
2.2 In vitro fermentation and biodegradation
The in vitro fermentation medium was prepared according to Goering and Van Soest [19]. A reducing solution containing sodium sulfide was added (2 mL) to the buffer shortly before rumen fluid addition. Ruminal inoculum (20 mL) and the buffer solution (80 mL) were mixed in each 250 mL bottle.
Ruminal inoculum was collected from the rumen of three sheep from a local slaughterhouse at Cairo (Egypt). Before slaughtering, sheep were ad libitum fed a diet containing concentrates, berseem hay, and RS at 500:400:100 (DM basis), with free access to water. The ruminal fluid was filtered through two-layered cheesecloth to remove large feed particles, and the particulate materials were squeezed to obtain microbes attached to feed particles. The initial pH of the inoculum was from 6.8 to 6.9. All replacement levels were tested in two incubation runs with 3 replicates in each run. In each incubation run, 2 bottles with inoculum but without feed (blanks) were also included to establish baseline fermentation GP.
A 1 g ± 10 mg sample for each TMR was weighed into filter bags (ANKOM F57; ANKOM Technology, Macedon, NY, USA) and then were placed into 250 mL ANKOM bottles (ANKOMRF Gas Production System) fitted with an automatic wireless in vitro GP module (ANKOM Technology, Macedon, NY, USA) with pressure sensors. The pressure was recorded every 10 min and cumulative pressure was calculated from these values. The gas pressure was converted into volume (mL) at standard pressure and temperature. The gas volume in the blank units was subtracted to obtain net GP. Values of GP at 0, 2, 4, 6, 8, 10, 12, 16, 20, 24, 36, 48, 72, and 96 h were extracted for estimation of GP kinetics. At each incubation time, gas samples (5 mL) were taken from the sampling vent and infused into a Gas-Pro detector (Gas Analyzer CROWCON Model Tetra3, Abingdon, UK) to measure the concentration of CH4 and CO2.
2.3 Sampling and analysis of fermentation variables
After 96 h of incubation, the fermentation was stopped by placing the bottles on ice for 5 min, and then pH was measured immediately using a pH meter. The ANKOM F57 filter bags were dried in a forced air oven at 55 °C for 48 h. Dry matter, NDF, and acid detergent fiber (ADF) degradation were calculated by subtracting the dried residue weight from the initial weight of dried substrate. Total gas and CH4 and CO2 productions were expressed in relation to degraded DM, NDF, and ADF at 96 h of incubation.
Samples (5 mL) of the supernatant fermented fluid from each bottle were taken in glass tubes for determination of ammonia-N (NH3-N) and total and individual VFA concentrations. A subsample of 3 mL was preserved with 3 mL of 0.2 M hydrochloric acid solution for measurement of NH3-N concentration according to AOAC [20]. An aliquot (0.8 mL) was mixed with 0.2 mL of metaphosphoric acid solution (250 g/L) for VFA analysis by steam distillation and titration method.
2.4 Chemical analysis
For evaluation of silage quality, silage samples (200 g fresh weight) were homogenized for 3 min with a laboratory blender after adding 800 mL of distilled water. The content was filtrated through 4 layers of cheesecloth and filtrate was assessed for pH using a digital pH meter (Thermo Scientific, Orion Star™ A121, Beverly, MA, USA), NH3-N [20], and VFA concentrations [20]. Aflatoxin (F1) concentration in silage was determined based on the standard methods [20] using a Fluorometer, Series-4 (VICAM, USA).
Samples of ingredients and formulated TMR were analyzed for ash after burning the samples in a muffle furnace at 550 °C for 12 h (method ID 942.05), CP using Kjeldahl method (method ID 954.01), and ether extract (EE) using diethyl ether in Soxhlet extractors (method ID 920.39) according to AOAC [20] methods. Neutral detergent fiber content was determined without alpha amylase but with sodium sulfite following the procedure of Van Soest et al. [21]. Acid detergent fiber content was analyzed according to AOAC [20] (method ID 973.18) and expressed exclusive of residual ash. Lignin was analyzed in the ADF residue according to Van Soest et al. [21]. Nonstructural carbohydrate, cellulose, hemicellulose, and organic matter (OM) concentrations were calculated.
2.5 Calculations and statistical analysis
For the estimation of GP kinetic, total gas and CH4 and CO2 productions (mL/g DM) data were fitted using the NLIN procedure of SAS (Version 9.4, SAS Inst., Inc., Cary, NC) according to France et al. [22] model as y = b × [1 − e−c (t − Lag)] where y is the volume of total GP and CH4 or CO2 productions at time t (h); b is the asymptotic GP (mL/g DM); c is the fractional rate of GP (/h), and Lag (h) is the discrete lag time before any GP.
Data were analyzed using the GLM procedure of SAS in a complete randomized design using the model: Yijk = μ + Ri + Dj + (R × D)ij + εijk where Yijk is the observation, μ is the population mean, Ri is the ration (diet or organic acid treatment) effect, Dj is the replacement level effect, (R × D)ij is the interaction between ration type and replacement level, and εijk is the residual error. Linear and quadratic contrasts were employed to determine the dose responses (increasing replacement levels) within diet.
3 Results
3.1 Chemical composition
Ensiling of DPL and RS with malic or lactic acids almost did not affect the concentrations of different nutrients compared to the ensiling without additives except for lower NDF content by lactic acid treatment (Table 1). Ensiling of DPL with malic or lactic acid decreased fermentation pH (P = 0.024) and NH3-N concentration (P = 0.006) while increased the concentration of VFA (P = 0.022); however, ensiling of RS did not affect silage measurements. Replacing berseem hay with different forms of treated DPL gradually decreased CP, EE, ADF, and cellulose contents and increased NDF and hemicellulose contents (Table 2).
3.2 Biogas production
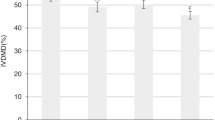
Figures 1, 2, and 3 show the in vitro ruminal total GP and CH4 and CO2 productions (mL/g incubated DM) of TMR containing different levels of DPL in different forms. Treatment × replacement level interactions were observed (P < 0.01) for the asymptotic total GP, the rate of total GP, the asymptotic CH4 production, the asymptotic CO2 production, CH4 and CO2 productions per unit degraded nutrients (i.e., mL/g degraded DM, mL/g degraded NDF, and mL/g degraded ADF), and proportional CH4 and CO2 productions (Tables 3 and 4). Diet type affected (P < 0.05) the asymptotic production of total gas, CH4 and CO2, the rate of total GP, production of CH4 and CO2 relative to nutrient degradation (mL/g degraded DM, mL/g degraded NDF, or mL/g degraded ADF), and proportional CH4 and CO2 productions. Replacement level affected (P < 0.05) the asymptotic production of total gas, CH4 and CO2, the rate of total gas and CO2 production, CH4 and CO2 productions (mL/g degraded DM, mL/g degraded NDF, and mL/g degraded ADF), and proportional CH4 and CO2 productions (Table 4).
Compared to the control, ensiling without additives decreased (P < 0.05) the asymptotic production of total gas, CH4 and CO2, and the rate of CH4 and CO2 productions while increased (P < 0.05) the lag time of CH4 and CO2 productions (Table 3). Replacement of berseem hay with incremental levels of lactic acid-treated DPL gradually decreased (linear effect, P < 0.01) the asymptotic production of total gas, CH4, and CO2. Replacement of berseem hay with malic acid-treated DPL also decreased the asymptotic total gas, CH4 and CO2 productions, the rates of total gas, CH4 and CO2 productions, and the lag time of total GP (Table 3). Ensiling of DPL with lactic acid (P < 0.001) and malic acid (P < 0.01) linearly decreased CH4 and CO2 productions (expressed as mL/g degraded DM, mL/g degraded NDF, or mL/g degraded ADF) (Table 4).
3.3 Degradability and fermentation
Ration × replacement interactions were observed (P < 0.05) for DM and ADF degradabilities, fermentation pH, and concentrations of total VFA and acetate, while ration (treatment) affected DM and ADF degradabilities and the concentrations of total VFA and acetate (Table 5). Replacement level affected (P < 0.05) DM and ADF degradabilities and the concentrations of total VFA, acetate, and butyrate.
Compared to the control, lactic acid-treated DPL quadratically decreased (P = 0.011) ADF degradability (lowest at 25%) without affecting DM or NDF degradabilities (Table 5). Replacing berseem hay with lactic acid-treated DPL linearly increased (P < 0.05) the concentration of total VFA and acetate. The replacement of berseem hay with malic acid-treated DPL did not affect the measured ruminal fermentation parameters.
4 Discussion
4.1 Chemical composition
Ensiling with malic or lactic acids usually did not affect the composition of DPL or RS, but decreased NDF content. This finding agrees the results of Kara [23] who observed that malic acid did not affect the composition of corn silage except decreased NDF contents. Similarly, Jiang et al. [17] reported that whole plant corn ensiled with organic acid mixture (acetic acid, propionic acid, and formic acid at a 1:2:7 ratio) applied at 6 mL/g fresh material reduced NDF content in the silage. The decreased fiber content in organic acid-treated silages may be due to hydrolysis of digestible cell wall fractions by the action of organic acids during ensiling process [17, 24].
The quality of ensiled materials differed between DPL and RS, which may be related to chemical composition and nature of fibers present in them. Silages of DPL were affected by malic or lactic acid administration. Malic and lactic acids administration during ensiling decreased fermentation pH, which indicates an improved silage quality. Additionally, the lowered NH3-N concentration is an indicator about less degradation rates of protein in the ensiled materials [25]. Lowering aflatoxin F1 during ensiling was previously observed [26] as a result of lowering ensiling pH. Aflatoxins are mainly produced by Aspergillus flavus and Aspergillus parasiticus, which are less tolerant to low pH in silages and anaerobic conditions [27].
4.2 Gas production
The significant treatment × replacement level interactions for measured GP parameters indicate that the effect of replacement levels is diet-dependent and that the recommended replacement level differs between different treatments. Elghangour et al. [7] indicated that the effect of organic acids on in vitro ruminal fermentation is diet-dependent. Parameters of GP showed significant differences among rations indicating mainly the effect of the additives as well as nutrient availability for ruminal microbes to ferment feeds [28].
Lactic acid treatments decreased the asymptotic total GP indicating negative effects on ruminal fermentation. Additionally, malic acid treatments decreased the asymptotic total GP and the rates of GP, while increased the lag time of GP. However, Kara [23] and El-Zaiat et al. [8] reported unaffected GP with in vitro inclusion of malic acid. Elghangour et al. [7] observed that administration of organic acids increased the asymptotic GP of diets with different maize silage to concentrate ratios. The negative effects in the present study may be related to the lowered pH in the organic acid treatments or indicate that the used levels of malic or lactic acids were high than recommended levels. Also, dose, diet, and type organic acids may be the reason for the inconsistency.
4.3 Methane and carbon dioxide productions
The significant treatment × replacement level interactions confirm the previous observations of other experiments that CH4 and CO2 productions are diet-dependent [7, 28]. In addition to polluting the environment, CH4 emissions cause animal energy losses and low production efficiency. In the present experiment, lactic and malic acid-treated DPL gradually decreased the asymptotic CH4 and CO2 productions and their rates of production indicating their use as an environmentally friendly strategy to reduce greenhouse gases mitigation. Organic acids (e.g., malic and lactic acids) can act as a hydrogen (H2) sink during their metabolic conversion to propionate, leading to decreased methanogenesis by [13, 29]. Additionally, DPL contains phenolic compounds [30, 31], which may lower CH4 production with increasing level of DPL. Phenolics including tannins can mitigate CH4 production by directly inhibiting methanogens, protozoa, and reduction of fiber degradation [32, 33]. In this study, CH4 production lowered per unit of degraded NDF, suggesting that at least a part of this reduced CH4 production may be attributed to the direct inhibition of protozoa or methanogens or both. El-Zaiat et al. [8] observed that in vitro inclusion of malic acid at 3 mg/g DM substrate decreased CH4 production by 33%. Similar results were observed with malic acid addition to corn silage [23].
4.4 Degradability and fermentation
Replacement of berseem hay with malic or lactic acid-treated DPL did not affect DM or NDF degradability. Similar results were observed by El-Zaiat et al. [8] and Genc et al. [34] who observed unaffected nutrient degradability with malic acid in vitro administration. In another experiment, Ebrahimi et al. [35] and Pal et al. [36] observed that fumaric or malic acids did not affect the digestibility of DM, OM, NDF, and ADF.
Greater propionate production promotes energy use for milk production and lactose synthesis [37], while increasing acetate is essential for milk fat concentration [38]. The inclusion of lactic acid-treated DPL in rations increased the concentration of total VFA, propionate, and acetate. Carro and Ranilla [39] observed that malic acid increased both of ruminal propionate and acetate concentration at the same time because malic acid can be converted to acetate and propionate following different pathways unlike other additives that can increase propionate at the expense of acetate. Abdelrahman et al. [12] noted that malic acid supplementation to growing lambs fed a concentrate diet numerically increased ruminal acetic acid and significantly decreased propionic acid concentration. Difference between diets, inclusion rate, and diet acid–base or diet buffering capacity in the present experiment and that of Abdelrahman et al. [12] may be the reason. It indicates that organic acid could perform better with diets containing forages. Organic acids may stimulate lactate utilization by Selenomonas ruminantium via the succinate–propionate pathway [40]. In the present study, pH values were, however, not changed by organic acid-treated DPL.
5 Conclusions
The treatment of date palm leaves with lactic acid or malic acid did not affect the nutrient composition of date palm leaves except decreased fiber content by lactic acid treatment. Replacing berseem hay with malic acid or lactic acid-treated date palm leaves decreased total gas and methane and carbon dioxide productions. The replacement of berseem hay with date palm leaves treated with lactic or malic acid is useful from an environmental view. Lactic acid treatment increased degradability of dry matter including fiber components and volatile fatty acid concentration, but methane decreased production to a greater extent compared with the malic acid treatment, which suggested that ensiling of date palm leaves with lactic acid is better suited than malic acid treatment. More research, both in vivo and in vitro experiments, is required to validate the observed results.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kholif AE, Elghandour MMY, Rodríguez GB et al (2017) Anaerobic ensiling of raw agricultural waste with a fibrolytic enzyme cocktail as a cleaner and sustainable biological product. J Clean Prod 142:2649–2655. https://doi.org/10.1016/j.jclepro.2016.11.012
Hamdon HA, Kholif AE, Mahmoud GB et al (2020) Enhancing the utilization of palm leaf hay using Bacillus subtilis and Phanerochaete chrysosporium in the diet of lambs under desert conditions. Ann Anim Sci 20:1395–1409. https://doi.org/10.2478/aoas-2020-0052
Kholif AE, Gouda GA, Patra AK (2022) The sustainable mitigation of in vitro ruminal biogas emissions by ensiling date palm leaves and rice straw with lactic acid bacteria and Pleurotus ostreatus for cleaner livestock production. J Appl Microbiol In press. https://doi.org/10.1111/jam.15432
Abu-Qaoud H (2015) Date palm status and perspective in Palestine. Springer, Netherlands
Medjekal S, Arhab R, Bousseboua H (2011) Nutritive value assessment of some desert by-products by gas production and rumen fermentation in vitro. Livest Res Rural Dev 23:200–209
Ziaei N, Hosseini SMMS (2009) Feeding value and in vitro digestibility of date-palm leaves supplemented with different supplementary energy. Pakistan J Biol Sci 12:817–820. https://doi.org/10.3923/pjbs.2009.817.820
Elghandour MMY, Kholif AE, Hernández A et al (2017) Effects of organic acid salts on ruminal biogas production and fermentation kinetics of total mixed rations with different maize silage to concentrate ratios. J Clean Prod 147:523–530. https://doi.org/10.1016/j.jclepro.2017.01.078
El-Zaiat HM, Kholif AE, Mohamed DA et al (2019) Enhancing lactational performance of Holstein dairy cows under commercial production: malic acid as an option. J Sci Food Agric 99:885–892. https://doi.org/10.1002/jsfa.9259
Sahoo A, Jena B (2014) Organic acids as rumen modifiers. Int J Sci Res 3:2262–2266
Martin SA, Streeter MN, Nisbet DJ et al (1999) Effects of DL-malate on ruminal metabolism and performance of cattle fed a high-concentrate diet. J Anim Sci 77:1008–1015. https://doi.org/10.2527/1999.7741008x
da Silva Dias MS, Ghizzi LG, Marques JA et al (2021) Effects of organic acids in total mixed ration and feeding frequency on productive performance of dairy cows. J Dairy Sci 104:5405–5416. https://doi.org/10.3168/jds.2020-19419
Abdelrahman MM, Alhidary I, Albaadani HH et al (2019) Effect of palm kernel meal and malic acid on rumen characteristics of growing Naemi lambs fed total mixed ration. Animals 9:408. https://doi.org/10.3390/ani9070408
Pal K, Patra AK, Sahoo A, Mandal GP (2014) Effect of nitrate and fumarate in Prosopis cineraria and Ailanthus excelsa leaves-based diets on methane production and rumen fermentation. Small Rumin Res 121:168–174. https://doi.org/10.1016/j.smallrumres.2014.08.004
Adesogan AT, Salawu MB (2004) Effect of applying formic acid, heterolactic bacteria or homolactic and heterolactic bacteria on the fermentation of bi-crops of peas and wheat. J Sci Food Agric 84:983–992. https://doi.org/10.1002/JSFA.1745
Zhang Y, Li D, Wang X et al (2019) Fermentation quality and aerobic stability of mulberry silage prepared with lactic acid bacteria and propionic acid. Anim Sci J 90:513–522. https://doi.org/10.1111/asj.13181
Kung L, Stokes MR, Lin CJ (2003) Silage additives. In: Buxton DR, Muck RE, Harrison JH (eds) Silage Science and Technology. Wiley, pp 305–360
Jiang F, Cheng H, Liu D, et al (2020) Treatment of whole-plant corn silage with lactic acid bacteria and organic acid enhances quality by elevating acid content, reducing pH, and inhibiting undesirable microorganisms. Front Microbiol 11:593088. https://doi.org/10.3389/fmicb.2020.593088
Abd El Tawab AM, Kholif AE, Hassan AM et al (2020) Feed utilization and lactational performance of Friesian cows fed beet tops silage treated with lactic acid bacteria as a replacement for corn silage. Anim Biotechnol 31:473–482. https://doi.org/10.1080/10495398.2019.1622556
Goering HK, Van Soest PJ (1975) Forage fiber analyses. ARS-USDA, Washington, USA, USA
AOAC (1997) Official methods of analysis, 16th edn. AOAC International, Washington DC
Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597. https://doi.org/10.3168/jds.S0022-0302(91)78551-2
France J, Dijkstra J, Dhanoa MS et al (2000) Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: derivation of models and other mathematical considerations. Br J Nutr 83:143–150. https://doi.org/10.1017/S0007114500000180
Kara K (2015) In vitro methane production and quality of corn silage treated with maleic acid. Ital J Anim Sci 14:718–722. https://doi.org/10.4081/ijas.2015.3994
Larsen SU, Hjort-Gregersen K, Vazifehkhoran AH, Triolo JM (2017) Co-ensiling of straw with sugar beet leaves increases the methane yield from straw. Bioresour Technol 245:106–115. https://doi.org/10.1016/j.biortech.2017.08.117
Sánchez-Duarte JI, García Á (2017) Ammonia-N concentration in alfalfa silage and its effects on dairy cow performance: a meta-analysis. Rev Colomb Ciencias Pecu 30:175–184. https://doi.org/10.17533/udea.rccp.v30n3a01
Li J, Wang W, Chen S et al (2021) Effect of lactic acid bacteria on the fermentation quality and mycotoxins concentrations of corn silage infested with mycotoxigenic fungi. Toxins 13:699. https://doi.org/10.3390/TOXINS13100699
Holmquist GU, Walker HW, Stahr HM (1983) Influence of temperature, pH, water activity and antifungal agents on growth of Aspergillus flavus and A. parasiticus. J Food Sci 48:778–782. https://doi.org/10.1111/j.1365-2621.1983.tb14897.x
Kholif AE, Elghandour MMY, Salem AZM et al (2017) The effects of three total mixed rations with different concentrate to maize silage ratios and different levels of microalgae Chlorella vulgaris on in vitro total gas, methane and carbon dioxide production. J Agric Sci 155:494–507. https://doi.org/10.1017/S0021859616000812
Newbold CJ, Rode LM (2006) Dietary additives to control methanogenesis in the rumen. Int Congr Ser 1293:138–147. https://doi.org/10.1016/j.ics.2006.03.047
Ziouti A, El Modafar C, Fleuriet A et al (1996) Phenolic compounds in date palm cultivars sensitive and resistant to Fusarium oxysporum. Biol Plant 38:451–457. https://doi.org/10.1007/BF02896679
Kriaa W, Fetoui H, Makni M et al (2012) Phenolic contents and antioxidant activities of date palm (Phoenix dactylifera L.) leaves. Int J Food Prop 15:1220–1232. https://doi.org/10.1080/10942912.2010.514673
Patra AK, Min BR, Saxena J (2012) Dietary tannins on microbial ecology of the gastrointestinal tract in ruminants. Dietary phytochemicals and microbes. Springer, Netherlands, pp 237–262
Patra AK (2016) Recent advances in measurement and dietary mitigation of enteric methane emissions in ruminants. Front Vet Sci 3:1–17. https://doi.org/10.3389/fvets.2016.00039
Genç B, Salman M, Bölükbaş B et al (2020) The effects of fumaric and malic acids on the in vitro true digestibility of some alternative feedstuffs for ruminants. Ankara Univ Vet Fak Derg 67:185–192. https://doi.org/10.33988/auvfd.623821
Ebrahimi SH, Datta MM, Heidarian V et al (2015) Effects of fumaric or malic acid and 9, 10 anthraquinone on digestibility, microbial protein synthesis, methane emission and performance of growing calves. Indian J Anim Sci 85:1000–1005
Pal K, Patra AK, Sahoo A, Soren NM (2015) Effects of nitrate and fumarate in tree leaves-based diets on nutrient utilization, rumen fermentation, microbial protein supply and blood profiles in sheep. Livest Sci 172:5–15
Evans JD, Martin SA (1997) Factors affecting lactate and malate utilization by Selenomonas ruminantium. Appl Environ Microbiol 63:4853–4858. https://doi.org/10.1128/aem.63.12.4853-4858.1997
Kholif AE, Kassab AY, Azzaz HH et al (2018) Essential oils blend with a newly developed enzyme cocktail works synergistically to enhance feed utilization and milk production of Farafra ewes in the subtropics. Small Rumin Res 161:43–50. https://doi.org/10.1016/j.smallrumres.2018.02.011
Carro MD, Ranilla MJ (2003) Effect of the addition of malate on in vitro rumen fermentation of cereal grains. Br J Nutr 89:181–188. https://doi.org/10.1079/BJN2002759
Martin SA, Sullivan HM, Evans JD (2000) Effect of sugars and malate on ruminal microorganisms. J Dairy Sci 83:2574–2579. https://doi.org/10.3168/jds.S0022-0302(00)75150-2
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This paper is based upon work supported by Science, Technology & Innovation Funding Authority (STDF) under grant: 41519.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was conducted in compliance with the guide of Agricultural Research and Teaching of Federation of Animal Science Societies, Champaign, IL, USA.
Consent to participate
All authors agree to participate in the current work.
Consent for publication
All authors agree to publish the findings of the current research.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Statement of novelty
Under semi-arid and arid conditions, trees and shrubs such as date palm can be used as an adequate source of feed for goats and sheep to reduce feed cost. However, the low nutritive value of such materials limits their utilization. Ensiling with organic acids can be used to enhance the nutritive value of date palm leaves and other agriculture byproducts before feeding to ruminants. Organic acid can improve the quality of silages produced from low-quality forages and byproducts, resulting in improved performance (daily gain or milk production) when these materials are fed to ruminants. This may enhance farmers’ gain and animal health. This is the first experiment to evaluate the ensiling of date palm leaves with malic or lactic acids to enhance its nutritive value as an unconventional feed.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kholif, A.E., Gouda, G.A., Morsy, T.A. et al. The effects of replacement of berseem hay in total mixed rations with date palm leaves ensiled with malic or lactic acids at different levels on the nutritive value, ruminal in vitro biogas production and fermentation. Biomass Conv. Bioref. 14, 3763–3775 (2024). https://doi.org/10.1007/s13399-022-02508-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02508-y