Abstract
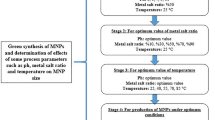
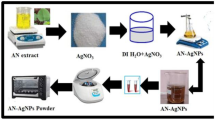
The traditional physical and chemical methods used in the production of silver nanoparticles (AgNP) consist of complex, expensive, and environmentally harmful processes. To overcome these problems, researchers have turned to the AgNP production method called green synthesis or biosynthesis. This study aims to produce AgNPs by green synthesis from the extracts of Ulva lactuca and Halopteris scoparia and to determine their antibacterial and antifungal activities. In this context, the effects of some process parameters on nanoparticle size were examined statistically and the antimicrobial activities of AgNPs produced under optimum conditions against various pathogenic bacteria and fungi were determined. The smallest AgNPs synthesized from Ulva lactuca and Halopteris scoparia extracts were measured as 40.19 ± 5.93 and 34.88 ± 4.38 nm, respectively, under the conditions of pH of 11, AgNO3 ratio of 10%, and temperature of 55 °C. As for the antibacterial activity of AgNPs, the diameters of the inhibition zones formed against pathogenic bacteria were found as between 10 and 15 mm. As for the antifungal activity, a reduction between 15 and 21.33 mm was observed in pathogenic fungi growths. With this study, it was concluded that macroalgae can be easily used in the green synthesis of AgNPs with antibacterial and antifungal properties. Future research of green-synthesized AgNPs from macroalgae will be highly important for being used as an antibacterial and antifungal agent in fields such as medicine and cosmetics.
Graphical abstract






Similar content being viewed by others
References
Wennersten R, Fidler J, Spitsyna A (2008) Nanotechnology: a new technological revolution in the 21st century. In: Handbook of performability engineering. pp 943–952
Mody V, Siwale R, Singh A, Mody H (2010) Introduction to metallic nanoparticles. J Pharm Bioallied Sci 2:282. https://doi.org/10.4103/0975-7406.72127
Sundararaju S, Arumugam M, Bhuyar P (2020) Microbacterium sp. MRS-1, a potential bacterium for cobalt reduction and synthesis of less/non-toxic cobalt oxide nanoparticles (Co3O4). Beni-Suef Univ J Basic Appl Sci 9:1–9. https://doi.org/10.1186/s43088-020-00070-y
Bhuyar P, Rahim MHA, Sundararaju S et al (2020) Synthesis of silver nanoparticles using marine macroalgae Padina sp. and its antibacterial activity towards pathogenic bacteria. Beni-Suef Univ J Basic Appl Sci 9:1–15. https://doi.org/10.1186/s43088-019-0031-y
Srikar SK, Giri DD, Pal DB et al (2016) Green synthesis of silver nanoparticles: a review. Green Sustain Chem 06:34–56. https://doi.org/10.4236/gsc.2016.61004
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9:385–406
Hasanin M, Al Abboud MA, Alawlaqi MM, et al (2021) Ecofriendly synthesis of biosynthesized copper nanoparticles with starch-based nanocomposite: antimicrobial, antioxidant, and anticancer activities. Biol Trace Elem Reshttps://doi.org/10.1007/s12011-021-02812-0
A. Hassaan M (2018) Green synthesis of Ag and Au nanoparticles from micro and macro algae - review. Int J Atmos Ocean Sci 2:10. https://doi.org/10.11648/j.ijaos.20180201.12
Hussain I, Singh NB, Singh A et al (2016) Green synthesis of nanoparticles and its potential application. Biotechnol Lett 38:545–560
Abdelghany TM, Al-Rajhi AMH, Al Abboud MA et al (2018) Recent advances in green synthesis of silver nanoparticles and their applications: about future directions. Rev Bionanoscience 8:5–16
Chandran SP, Chaudhary M, Pasricha R et al (2006) Synthesis of gold nanotriangles and silver nanoparticles using aloe vera plant extract. Biotechnol Prog 22:577–583. https://doi.org/10.1021/bp0501423
Ahmed S, Saifullah AM et al (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci 9:1–7. https://doi.org/10.1016/j.jrras.2015.06.006
Ping Y, Zhang J, Xing T et al (2018) Green synthesis of silver nanoparticles using grape seed extract and their application for reductive catalysis of Direct Orange 26. J Ind Eng Chem 58:74–79. https://doi.org/10.1016/j.jiec.2017.09.009
Saxena J, Sharma PK, Sharma MM, Singh A (2016) Process optimization for green synthesis of silver nanoparticles by Sclerotinia sclerotiorum MTCC 8785 and evaluation of its antibacterial properties. Springerplus 5:861. https://doi.org/10.1186/s40064-016-2558-x
Koilparambil D, Kurian LC, Vijayan S, Manakulam Shaikmoideen J (2016) Green synthesis of silver nanoparticles by Escherichia coli : analysis of antibacterial activity. J Water Environ Nanotechnol 1:63–74. https://doi.org/10.7508/jwent.2016.01.008
Kannan RRR, Stirk WA, Van Staden J (2013) Synthesis of silver nanoparticles using the seaweed Codium capitatum P.C. Silva (Chlorophyceae). South African J Bot 86:1–4. https://doi.org/10.1016/j.sajb.2013.01.003
Vijayan SR, Santhiyagu P, Singamuthu M, et al (2014) Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, Turbinaria conoides, and their antimicrofouling activity. Sci World J 2014https://doi.org/10.1155/2014/938272
Jung KA, Lim SR, Kim Y, Park JM (2013) Potentials of macroalgae as feedstocks for biorefinery. Bioresour Technol 135:182–190. https://doi.org/10.1016/j.biortech.2012.10.025
Koçer AT, Özçimen D (2021) Determination of combustion characteristics and kinetic parameters of Ulva lactuca and its biochar. Biomass Convers Biorefinery.https://doi.org/10.1007/s13399-020-01245-4
Bird MI, Wurster CM, de Paula Silva PH et al (2011) Algal biochar - production and properties. Bioresour Technol 102:1886–1891. https://doi.org/10.1016/j.biortech.2010.07.106
Kulkarni N, Muddapur U (2014) Biosynthesis of metal nanoparticles: a review. J. Nanotechnol. 2014
Dubois M, Gilles KA, Hamilton JK et al (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol. https://doi.org/10.1139/o59-099
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin. J Biol Chemhttps://doi.org/10.1016/0304-3894(92)87011-4
Safafar H, Van WJ, Møller P, Jacobsen C (2015) Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar Drugs 13:7339–7356. https://doi.org/10.3390/md13127069
García R, Pizarro C, Lavín AG, Bueno JL (2012) Characterization of Spanish biomass wastes for energy use. Bioresour Technol 103:249–258. https://doi.org/10.1016/j.biortech.2011.10.004
Ramli ANM, Badrulzaman SZS, Hamid HA, Bhuyar P (2021) Antibacterial and antioxidative activity of the essential oil and seed extracts of Artocarpus heterophyllus for effective shelf-life enhancement of stored meat. J Food Process Preserv 45https://doi.org/10.1111/jfpp.14993
Bhuyar P, Rahim MH, Sundararaju S et al (2020) Antioxidant and antibacterial activity of red seaweed; Kappaphycus alvarezii against pathogenic bacteria. Glob J Environ Sci Manag 6:47–58. https://doi.org/10.22034/gjesm.2020.01.04
Vehapi M, Koçer AT, Yılmaz A, Özçimen D (2020) Investigation of the antifungal effects of algal extracts on apple-infecting fungi. Arch Microbiol 202:455–471. https://doi.org/10.1007/s00203-019-01760-7
Khairy HM, El-Sheikh MA (2015) Antioxidant activity and mineral composition of three Mediterranean common seaweeds from Abu-Qir Bay. Egypt Saudi J Biol Sci 22:623–630. https://doi.org/10.1016/j.sjbs.2015.01.010
Prasedya ES, Martyasari NWR, Apriani R, et al (2019) Antioxidant activity of Ulva lactuca L. from different coastal locations of Lombok Island, Indonesia. In: AIP Conference Proceedings. p 20003
Anigol LB, Gurubasavaraj PM, Charantimath JS (2017) Effect of concentration and pH on the size of silver nanoparticles synthesized by green chemistry. Org Med Chem Int J Biosynth 3:1–5
Vanaja M, Rajeshkumar S, Paulkumar K et al (2013) Kinetic study on green synthesis of silver nanoparticles using Coleus aromaticus leaf extract. Pelagia Res Libr 4:50–55
Dubey SP, Lahtinen M, Sillanpää M (2010) Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem 45:1065–1071. https://doi.org/10.1016/j.procbio.2010.03.024
Philip D (2010) Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim Acta - Part A Mol Biomol Spectrosc 77:807–810. https://doi.org/10.1016/j.saa.2010.08.008
Jagajjanani Rao K, Paria S (2013) Green synthesis of silver nanoparticles from aqueous Aegle marmelos leaf extract. Mater Res Bull 48:628–634. https://doi.org/10.1016/j.materresbull.2012.11.035
Pourmortazavi SM, Taghdiri M, Makari V, Rahimi-Nasrabadi M (2015) Procedure optimization for green synthesis of silver nanoparticles by aqueous extract of Eucalyptus oleosa. Spectrochim Acta - Part A Mol Biomol Spectrosc 136:1249–1254. https://doi.org/10.1016/j.saa.2014.10.010
Rodríguez-León E, Iñiguez-Palomares R, Navarro RE et al (2013) Synthesis of silver nanoparticles using reducing agents obtained from natural sources (Rumex hymenosepalus extracts). Nanoscale Res Lett 8:1–9. https://doi.org/10.1186/1556-276X-8-318
Khan M, Khan M, Adil SF et al (2013) Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int J Nanomedicine 8:1507–1516. https://doi.org/10.2147/IJN.S43309
Sivagnanam SP, Tilahun Getachew A, Choi JH et al (2017) Green synthesis of silver nanoparticles from deoiled brown algal extract via Box-Behnken based design and their antimicrobial and sensing properties. Green Process Synth 6:147–160. https://doi.org/10.1515/gps-2016-0052
Kahrilas GA, Wally LM, Fredrick SJ et al (2014) Microwave-assisted green synthesis of silver nanoparticles using orange peel extract. ACS Sustain Chem Eng 2:367–376. https://doi.org/10.1021/sc4003664
Safekordi AA, Attar H, Ghorbani HR (2011) Optimization of silver nanoparticles production by E.coli and the study of reaction kinetics. In: International Conference on Chemical, Ecology and Environmental Sciences (ICCEES’2011). pp 346–350
Kredy HM (2018) The effect of pH, temperature on the green synthesis and biochemical activities of silver nanoparticles from Lawsonia inermis extract. J Pharm Sci Res 10:2022–2026
Sun Q, Cai X, Li J et al (2014) Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surfaces A Physicochem Eng Asp 444:226–231. https://doi.org/10.1016/j.colsurfa.2013.12.065
Ibrahim HMM (2019) Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci 8:265–275. https://doi.org/10.1016/j.jrras.2015.01.007
Koçer AT, Mutlu B, Özçimen D (2020) Investigation of biochar production potential and pyrolysis kinetics characteristics of microalgal biomass. Biomass Convers Biorefinery 10:85–94. https://doi.org/10.1007/s13399-019-00411-7
Bhakya S, Muthukrishnan S, Sukumaran M, Muthukumar M (2016) Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl Nanosci 6:755–766. https://doi.org/10.1007/s13204-015-0473-z
Vanaja M, Annadurai G (2013) Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl Nanosci 3:217–223. https://doi.org/10.1007/s13204-012-0121-9
Anandalakshmi K, Venugobal J, Ramasamy V (2016) Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci 6:399–408. https://doi.org/10.1007/s13204-015-0449-z
Punyamurthy R, Sampathkumar D, Ranganagowda RPG et al (2017) Mechanical properties of abaca fiber reinforced polypropylene composites: effect of chemical treatment by benzenediazonium chloride. J King Saud Univ - Eng Sci 29:289–294. https://doi.org/10.1016/j.jksues.2015.10.004
Koçer AT, Özçimen D (2018) Investigation of the biogas production potential from algal wastes. Waste Manag Res 36:1100–1105. https://doi.org/10.1177/0734242X18798447
Socrates G (2001) Infrared and Raman characteristic group frequencies. Tables and charts. J Raman Spectrosc 347
Ramli ANM, Manap NWA, Bhuyar P, Azelee NIW (2020) Passion fruit (Passiflora edulis) peel powder extract and its application towards antibacterial and antioxidant activity on the preserved meat products. SN Appl Sci 2:1–11. https://doi.org/10.1007/s42452-020-03550-z
Arifin DCV, Saragih DI, Santosa SJ (2020) Antibacterial activity of silver nanoparticles synthesized using tyrosine as capping and reducing agent. Int J Emerg Trends Eng Res 8:2414–2421. https://doi.org/10.30534/ijeter/2020/34862020
Khan Z, Al-Thabaiti SA, Obaid AY, Al-Youbi AO (2011) Preparation and characterization of silver nanoparticles by chemical reduction method. Colloids Surfaces B Biointerfaces 82:513–517. https://doi.org/10.1016/j.colsurfb.2010.10.008
Feng QL, Wu J, Chen GQ et al (2000) A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res 52:662–668. https://doi.org/10.1002/1097-4636(20001215)52:4%3c662::AID-JBM10%3e3.0.CO;2-3
Salman HD (2017) Evaluation and comparison the antibacterial activity of silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on some pathogenic bacteria. J Glob Pharma Technol 9:238–248
Anjali KP, Sangeetha BM, Devi G et al (2019) Bioprospecting of seaweeds (Ulva lactuca and Stoechospermum marginatum): the compound characterization and functional applications in medicine-a comparative study. J Photochem Photobiol B Biol 200:111622. https://doi.org/10.1016/j.jphotobiol.2019.111622
Tan SP, O’Sullivan L, Prieto ML et al (2012) Extraction and bioautographic-guided separation of antibacterial compounds from Ulva lactuca. J Appl Phycol 24:513–523. https://doi.org/10.1007/s10811-011-9747-3
Bhuyar P, Rahim MHA, Maniam GP et al (2020) Exploration of bioactive compounds and antibacterial activity of marine blue-green microalgae (Oscillatoria sp.) isolated from coastal region of west Malaysia. SN Appl Sci 2:1–10. https://doi.org/10.1007/s42452-020-03698-8
Dhand V, Soumya L, Bharadwaj S et al (2016) Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater Sci Eng C 58:36–43. https://doi.org/10.1016/j.msec.2015.08.018
Devi JS, Bhimba BV (2014) Antibacterial and antifungal activity of silver nanoparticles synthesized using Hypnea muciformis. Biosci Biotechnol Res Asia 11:235–238. https://doi.org/10.13005/bbra/1260
Mohamed El-Kadi S, Kamel Mahmoud M, Abd-Elfattah Sayed-Ahmed K, et al (2018) Comparison between silver nanoparticles and silver nitrate as antifungal agent. Int J Nanosci Nanoeng 4:5–11
Ghojavand S, Madani M, Karimi J (2020) Green synthesis, characterization and antifungal activity of silver nanoparticles using stems and flowers of felty germander. J Inorg Organomet Polym Mater 30:2987–2997. https://doi.org/10.1007/s10904-020-01449-1
Kumavat SR, Mishra S (2021) Green synthesis of silver nanoparticles using Borago officinalis leaves extract and screening its antimicrobial and antifungal activity. Int Nano Lett 1:1–16. https://doi.org/10.1007/s40089-021-00345-x
Balashanmugam P, Balakumaran MD, Murugan R et al (2016) Phytogenic synthesis of silver nanoparticles, optimization and evaluation of in vitro antifungal activity against human and plant pathogens. Microbiol Res 192:52–64. https://doi.org/10.1016/j.micres.2016.06.004
Rout Y (2012) Green synthesis of silver nanoparticles using Ocimum sanctum (Tulashi) and study of their antibacterial and antifungal activities. J Microbiol Antimicrob 4:103–109. https://doi.org/10.5897/jma11.060
Yousaf H, Mehmood A, Ahmad KS, Raffi M (2020) Green synthesis of silver nanoparticles and their applications as an alternative antibacterial and antioxidant agents. Mater Sci Eng C 112:110901. https://doi.org/10.1016/j.msec.2020.110901
Alkhulaifi MM, Alshehri JH, Alwehaibi MA et al (2020) Green synthesis of silver nanoparticles using Citrus limon peels and evaluation of their antibacterial and cytotoxic properties. Saudi J Biol Sci 27:3434–3441. https://doi.org/10.1016/j.sjbs.2020.09.031
Dutta T, Ghosh NN, Das M et al (2020) Green synthesis of antibacterial and antifungal silver nanoparticles using Citrus limetta peel extract: experimental and theoretical studies. J Environ Chem Eng 8:104019. https://doi.org/10.1016/j.jece.2020.104019
Abdelghany TM, Hassan MM, El-Naggar MA (2020) GC/MS analysis of Juniperus procera extract and its activity with silver nanoparticles against Aspergillus flavus growth and aflatoxins production. Biotechnol Reports 27:https://doi.org/10.1016/j.btre.2020.e00496
Bakri MM, El-Naggar MA, Helmy EA et al (2020) Efficacy of Juniperus procera constituents with silver nanoparticles against Aspergillus fumigatus and Fusarium chlamydosporum. Bionanoscience 10:62–72. https://doi.org/10.1007/s12668-019-00716-x
Acknowledgements
The authors would like to acknowledge that this paper is submitted in partial fulfillment of the requirements for the Ph.D. degree at Yildiz Technical University. Anıl Tevfik Koçer received financial support from The Scientific and Technological Research Council of Turkey (TUBITAK – BIDEB 2211/A National Scholarship Programme for Ph.D. Students) and 100/2000 The Council of Higher Education (YÖK) Doctorate Scholarship Programme. We would like to thank Meyrem Vehapi who helped to carry out the antimicrobial analysis test.
Funding
This work was supported by the Yıldız Technical University Scientific Research Project Coordination Unit (Project number: FDK-2019-3816).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koçer, A.T., Özçimen, D. Eco-friendly synthesis of silver nanoparticles from macroalgae: optimization, characterization and antimicrobial activity. Biomass Conv. Bioref. (2022). https://doi.org/10.1007/s13399-022-02506-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-022-02506-0