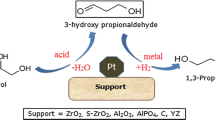
Abstract
Investigations on the metal phosphate (MP: M = Al, Ti, Zr and Nb) solid acid catalysts are carried out in the liquid phase through the acetylation of glycerol in acetic acid and acetalization in acetone. Hydrothermal procedure was employed in the preparation of these catalysts. The physico-chemical properties, viz; amorphous or crystalline nature is determined by using X-ray diffraction, pore size distribution is measured from their respective N2 adsorption–desorption isotherms, stretching and bending modes of various molecules is found using FT-IR and charge transfer transitions are determined using UV-DRS of these catalysts. Studies of adsorbed pyridine using ex situ FT-IR analysis and NH3-temperature programmed desorption are also carried out on these catalysts to get an insight into the structural and acidic properties by varying the metal component. The functionality of glycerol acetylation and acetalization is explained in terms of the acidity and structural properties of these MP catalysts. From the results, it was observed that the NbP was the most active catalyst, and it was achieved the highest conversion of glycerol in both acylation and acetalization reactions with 89% and 92% respectively. However, in acylation and acetylation reactions, we observed 21%, 54% and 25% selectivities for mono, di and triacetin respectively and 100% selectivity of solketal respectively for NbP catalyst. It was also observed that NbP was found to the best catalyst for activity and selectivity in both reactions as compared to TiP, ZrP and AlP. It is noteworthy to mention here is that both catalytic reactions were significantly affected by the material properties such as total acidity and textural properties. However, the various reaction parameters such as reaction temperature, mole ratio of glycerol to acetic acid, reaction time, and catalyst loadings also have some influence on the catalytic activity results.











Similar content being viewed by others
Data availability
Not applicable.
References
Khezerlou HS, Vahdani B, Yazdani M (2021) Designing a resilient and reliable biomass-to-biofuel supply chain under risk pooling and congestion effects and fleet management. J Clean Prod 281:125101. https://doi.org/10.1016/j.jclepro.2020.125101
Rashid T, Ali Ammar Taqvi S, Sher F et al (2021) Enhanced lignin extraction and optimisation from oil palm biomass using neural network modelling. Fuel 293:120485. https://doi.org/10.1016/j.fuel.2021.120485
Wu F, Jiang H, Zhu X et al (2021) Effect of tungsten psecies on selective hydrogenolysis of glycerol to 1,3-propanediol. ChemSusChem 14:569–581. https://doi.org/10.1002/cssc.202002405
Dodekatos G, Schünemann S, Tüysüz H (2018) Recent advances in thermo-, photo-, and electrocatalytic glycerol oxidation. ACS Catal 8:6301–6333. https://doi.org/10.1021/acscatal.8b01317
Lemos COT, Rade LL, Barrozo MADS et al (2017) Optimization of catalytic glycerol etherification with ethanol in a continuous reactor. Energy and Fuels 31:5158–5164. https://doi.org/10.1021/acs.energyfuels.7b00194
Rao GS, Rajan NP, Pavankumar V, Chary KVR (2014) Vapour phase dehydration of glycerol to acrolein over NbOPO4 catalysts. J Chem Technol Biotechnol 89:1890–1897. https://doi.org/10.1002/jctb.4273
Ginjupalli S, Balla P, Shaik H et al (2019) Comparative study of vapour phase glycerol dehydration over different tungstated metal phosphate acid catalysts. New J Chem 43:16860–16869. https://doi.org/10.1039/c9nj04484a
Srinivasa Rao G, Hussain S, Chary KVR (2018) Porous zirconium phosphate solid acid catalysts with variable Zr/P ratio for gas phase glycerol dehydration to acrolein. Mater Today Proc 5:25773–25781. https://doi.org/10.1016/j.matpr.2018.06.569
Ammaji S, Rao GS, Chary KVR (2018) Acetalization of glycerol with acetone over various metal-modified SBA-15 catalysts. Appl Petrochemical Res 8:107–118. https://doi.org/10.1007/s13203-018-0197-6
Gadamsetti S, Rajan NP, Rao GS, Chary KVR (2015) Acetalization of glycerol with acetone to bio fuel additives over supported molybdenum phosphate catalysts. Elsevier B.V
Fokum E, Zabed HM, Ravikumar Y, et al (2021) Co-fermentation of glycerol and sugars by Clostridium beijerinckii: enhancing the biosynthesis of 1,3-propanediol. Food Biosci 41. https://doi.org/10.1016/j.fbio.2021.101028
Ebadi Pour N, Dumeignil F, Katryniok B, et al (2021) Investigating the active phase of Ca-based glycerol polymerization catalysts: on the importance of calcium glycerolate. Mol Catal 507. https://doi.org/10.1016/j.mcat.2021.111571
Raemas AFA, Cahyonugroho J, Wicaksono AD et al (2021) Ultrasound-assisted esterification of glycerol for triacetin production. IOP Conf Ser Mater Sci Eng 1053:012062. https://doi.org/10.1088/1757-899x/1053/1/012062
Nda-Umar UI, Ramli I, Taufiq-Yap YH, Muhamad EN (2019) An overview of recent research in the conversion of glycerol into biofuels, fuel additives and other bio-based chemicals. Catalysts 9. https://doi.org/10.3390/catal9010015
Melero JA, Vicente G, Morales G et al (2010) Oxygenated compounds derived from glycerol for biodiesel formulation: influence on en 14214 quality parameters. Fuel 89:2011–2018. https://doi.org/10.1016/j.fuel.2010.03.042
Wang S, Guin JA (2000) Silica-supported sulfated zirconia: a new effective acid solid for etherification. Chem Commun 2499–2500. https://doi.org/10.1039/b007475f
Al-Rawi UA, Sher F, Hazafa A et al (2020) Catalytic activity of Pt loaded zeolites for hydroisomerization of n-hexane using supercritical CO2. Ind Eng Chem Res 59:22092–22106. https://doi.org/10.1021/acs.iecr.0c05184
Reddy PS, Sudarsanam P, Raju G, Reddy BM (2010) Synthesis of bio-additives: acetylation of glycerol over zirconia-based solid acid catalysts. Catal Commun 11:1224–1228. https://doi.org/10.1016/j.catcom.2010.07.006
Srinivasa Rao G, Pethan Rajan N, Hari Sekhar M et al (2014) Porous zirconium phosphate supported tungsten oxide solid acid catalysts for the vapour phase dehydration of glycerol. J Mol Catal A Chem 395:486–493. https://doi.org/10.1016/j.molcata.2014.09.018
Rao GS, Kumar BP, Hussain S, Bhanuchander P (2020) Metal phosphates: preparation, characterization and catalytic evolution for vapour phase dehydration of glycerol to acrolein. Adv Sci Eng Med 12:356–363. https://doi.org/10.1166/asem.2020.2507
Costa MCC, Johnstone RAW, Whittaker D (1995) Properties of polymeric zirconium phosphates as Friedel-crafts catalysts. J Mol Catal A Chem 103:155–162. https://doi.org/10.1016/1381-1169(95)00123-9
La Ginestra A, Patrono P, Berardelli ML et al (1987) Catalytic activity of zirconium phosphate and some derived phases in the dehydration of alcohols and isomerization of butenes. J Catal 103:346–356. https://doi.org/10.1016/0021-9517(87)90126-6
Lindblad T, Rebenstorf B, Yan ZG, Andersson SLT (1994) Characterization of vanadia supported on amorphous AlPO4 and its properties for oxidative dehydrogenation of propane. Appl Catal A, Gen 112:187–208. https://doi.org/10.1016/0926-860X(94)80219-X
Fei H, Zhou X, Zhou H et al (2007) Facile template-free synthesis of meso-macroporous titanium phosphate with hierarchical pore structure. Microporous Mesoporous Mater 100:139–145. https://doi.org/10.1016/j.micromeso.2006.10.019
Altaf F, Niazi MBK, Jahan Z et al (2021) Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J Polym Environ 29:156–174. https://doi.org/10.1007/s10924-020-01866-w
Jubeen F, Liaqat A, Sultan M et al (2019) Green synthesis and biological evaluation of novel 5-fluorouracil derivatives as potent anticancer agents. Saudi Pharm J 27:1164–1173. https://doi.org/10.1016/j.jsps.2019.09.013
Giovanni ER, John MW, Christopher JM, et al (2020) Phosgene formation via carbon monoxide and dichlorine reaction over an activated carbon catalyst: reaction testing arrangements. Applied Catalysis A, General 594: 117467. https://doi.org/10.1016/j.apcata.2020.117467
Feng W, Jean-Luc D, Wataru U, et al (2009) Catalytic dehydration of glycerol over vanadium phosphate oxides in the presence of molecular oxygen. J Catalysis 268: 260–267. https://doi.org/10.1016/j.jcat.2009.09.024
Mitta H, Devunuri N, Sunkari J et al (2021) A highly active dispersed copper oxide phase on calcined Mg9Al2.7-Ga2.3O2 catalysts in glycerol hydrogenolysis. Catal Today 375:204–215. https://doi.org/10.1016/j.cattod.2020.02.032
Dutta A, Patra AK, Dutta S et al (2012) Hierarchically porous titanium phosphate nanoparticles: an efficient solid acid catalyst for microwave assisted conversion of biomass and carbohydrates into 5-hydroxymethylfurfural. J Mater Chem 22:14094–14100. https://doi.org/10.1039/c2jm30623a
Bhaumik A, Inagaki S (2001) Mesoporous titanium phosphate molecular-sieves with ion-exchange capacity. J Am Chem Soc 123:691–696. https://doi.org/10.1021/ja002481s
Kim HN, Keller SW, Mallouk TE et al (1997) Characterization of zirconium phosphate/polycation thin films grown by sequential adsorption reactions. Chem Mater 9:1414–1421. https://doi.org/10.1021/cm970027q
Mercado A, Farfán-Torres EM, Regazzoni AE, Blesa MA (2003) Electrophoretic behavior of α-Zr(HPO4)2 and its phosphonated derivatives. Colloids Surfaces A Physicochem Eng Asp 218:277–284. https://doi.org/10.1016/S0927-7757(03)00002-5
Sinhamahapatra A, Sutradhar N, Roy B et al (2010) Mesoporous zirconium phosphate catalyzed reactions: synthesis of industrially important chemicals in solvent-free conditions. Appl Catal A Gen 385:22–30. https://doi.org/10.1016/j.apcata.2010.06.016
De la Cruz MHC, Da Silva JFC, Lachter ER (2003) Liquid phase benzylation of aromatic compounds with benzyl alcohol catalyzed by niobium phosphate. Appl Catal A Gen 245:377–382. https://doi.org/10.1016/S0926-860X(02)00639-7
Tchenar YN, Choukchou-Braham A, Bachir R (2012) RuO 2 supported on V 2O 5-Al 2O 3 material as heterogeneous catalyst for cyclohexane oxidation reaction. Bull Mater Sci 35:673–681. https://doi.org/10.1007/s12034-012-0331-5
Mal NK, Fujiwara M (2002) Synthesis of hexagonal and cubic super-microporous niobium phosphates with anion exchange capacity and catalytic properties. Chem Commun 2:2702–2703. https://doi.org/10.1039/b207976c
Patel SM, Chudasama UV, Ganeshpure PA (2003) Ketalization of ketones with diols catalyzed by metal(IV) phosphates as solid acid catalysts. J Mol Catal A Chem 194:267–271. https://doi.org/10.1016/S1381-1169(02)00538-1
Pagliaro M, Ciriminna R, Kimura H et al (2007) From glycerol to value-added products. Angew Chemie - Int Ed 46:4434–4440. https://doi.org/10.1002/anie.200604694
Freese U, Heinrich F, Roessner F (1999) Acylation of aromatic compounds on H-Beta zeolites. Catal Today 49:237–244. https://doi.org/10.1016/S0920-5861(98)00429-5
Gonçalves VLC, Pinto BP, Silva JC, Mota CJA (2008) Acetylation of glycerol catalyzed by different solid acids. Catal Today 133–135:673–677. https://doi.org/10.1016/j.cattod.2007.12.037
Silva LN, Gonçalves VLC, Mota CJA (2010) Catalytic acetylation of glycerol with acetic anhydride. Catal Commun 11:1036–1039. https://doi.org/10.1016/j.catcom.2010.05.007
Reguera FM, de Araujo LRR, Picardo MC et al (2004) The use of niobium based catalysts for liquid fuel production. Mater Res 7:343–348. https://doi.org/10.1590/s1516-14392004000200021
Da Silva Ferreira AC, Barbe JC, Bertrand A (2002) Heterocyclic acetals from glycerol and acetaldehyde in port wines: evolution with aging. J Agric Food Chem 50:2560–2564. https://doi.org/10.1021/jf011391j
Acknowledgements
The authors thank the University of Technology and Applied Sciences for the support to encourage research. There is no funding involved in support to the present research. Finally, the anonymous reviewers are gratefully acknowledged for their constructive comments on the paper.
Author information
Authors and Affiliations
Contributions
SRG: Conception and design of the work, data analysis and interpretation, drafting the article and final approval of the version to be published. PKB: Characterization of samples, data analysis and interpretation and final approval of the version to be published. RP: Critical revision of the article and final approval of the version to be published. PRN: Critical revision of the article and final approval of the version to be published. RP: Helping in manuscript preparation during first and second revision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ginjupalli, S.R., Balla, P., C., R.P. et al. Acid catalysed glycerol transformation to fuel additives over different metal phosphate solid acid catalysts. Biomass Conv. Bioref. 13, 12749–12761 (2023). https://doi.org/10.1007/s13399-021-02259-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-02259-2