Abstract
Kappaphycus alvarezii reject (KR) and solid food waste (SFW) are unused sources of carbohydrates; the production of bioethanol from these raw materials has not yet been reported by any researchers so far. The present study was conducted to optimize the fermentation parameters using RSM (Design-Expert version 7.0 software). KR and SFW were fermented by using Saccharomyces cerevisiae for bioethanol production. Logistic and modified Gompertz kinetic models were fitted fermentation time against bioethanol yield data. The gas chromatography flame ionization detector (GC–FID) was used for bioethanol confirmation. The optimum conditions for an incubation time of 24 h, inoculum size of 15 vol%, and agitation speed of 90 rpm at pH 5 were predicted by RSM. Under these experimental conditions, the best yield of bioethanol was 68% (w/w), which is in good agreement with the predicted value from RSM of 70% (w/w) with an R2 of 0.97. Under the optimized conditions, the reducing sugar reduced from 30.83 to 8.55 g/L with a conversion efficiency of 70%. Overall, KR and SFW were effective resources for the production of bioethanol to meet the future energy demand. The diversion of SFW through their study will provide a breakthrough for the reduction of energy potential SFW to landfills, contributing to the climate change initiative.
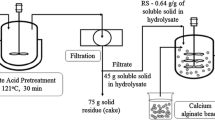
Graphical abstract





























Similar content being viewed by others
References
Manilal A, Sujith S, Kiran GS, Selvin J, Shakir C, Gandhimathi R, Panikkar MVN (2009) Biopotentials of seaweeds collected from southwest coast of India. J Mar Sci Technol 17:67–73
Sudhakar MP, Merlyn R, Arunkumar K, Perumal K (2016) Characterization, pretreatment and saccharification of spent seaweed biomass for bioethanol production using baker’s yeast. Biomass Bioenerg 90:148–154
Packiyadhas P, Shanmuganantham Selvanantham D (2020) Compositional and structural evaluation of Kappaphycus alvarezii rejects and solid food waste blends for bio ethanol production. Energy Sources Part A Recover Util Environ Eff 42:1–17
Khambhaty Y, Mody K, Gandhi MR, Thampy S, Maiti P, Brahmbhatt H, Eswaran K, Ghosh PK (2012) Kappaphycus alvarezii as a source of bioethanol. Bioresour Technol 103:180–185
Wang Q, Ma H, Wang X, Ji Y (2004) Resource recycling technology of food wastes. Mod Chem Ind 24:56–59
Kiran EU, Liu Y (2015) Bioethanol production from mixed food waste by an effective enzymatic pretreatment. Fuel 159:463–469
Tanaka M, Ozaki H, Ando A, Kambara S, Moritomi H (2008) Basic characteristics of food waste and food ash on steam gasification. Ind Eng Chem Res 47:2414–2419
Okonko IO, Adeola OT, Aloysius FE, Damilola AO, Adewale OA (2009) Utilization of food wastes for sustainable development. Electron J Environ Agric Food Chem 8:263–286
McMillan JD (1997) Bioethanol production: status and prospects. Renew Energy 10:295–302
Islam MN, Park K-J, Yoon H-S (2012) Methane production potential of food waste and food waste mixture with swine manure in anaerobic digestion. J Biosyst Eng 37:100–105
Zhu T, Curtis J, Clancy M (2019) Promoting agricultural biogas and biomethane production: Lessons from cross-country studies. Renew Sustain Energy Rev 114:109332
Qyyum MA, Haider J, Qadeer K, Valentina V, Khan A, Yasin M, Aslam M, De Guido G, Pellegrini LA, Lee M (2020) Biogas to liquefied biomethane: assessment of 3P’s–production, processing, and prospects. Renew Sustain Energy Rev 119:109561
Hou T, Zhao J, Lei Z, Shimizu K, Zhang Z (2020) Synergistic effects of rice straw and rice bran on enhanced methane production and process stability of anaerobic digestion of food waste. Bioresour Technol 314:123775
Krithiga T, Sabina XJ, Rajesh B, Ilbeygi H, Shetty AN, Reddy R, Karthikeyan J (2018) Cooked food waste—an efficient and less expensive precursor for the generation of activated carbon. J Nanosci Nanotechnol 18:4106–4113
Sophanodorn K, Unpaprom Y, Whangchai K, Duangsuphasin A, Manmai N, Ramaraj R (2020) A biorefinery approach for the production of bioethanol from alkaline-pretreated, enzymatically hydrolyzed Nicotiana tabacum stalks as feedstock for the bio-based industry. Biomass Convers Biorefinery 2:1–9
Casabar JT, Unpaprom Y, Ramaraj R (2019) Fermentation of pineapple fruit peel wastes for bioethanol production. Biomass Convers Biorefinery 9:761–765
Kadimpati KK, Thadikamala S, Devarapalli K, Banoth L, Uppuluri KB (2021) Characterization and hydrolysis optimization of Sargassum cinereum for the fermentative production of 3G bioethanol. Biomass Convers Biorefinery 2:1–11
Sudhakar MP, Arunkumar K, Perumal K (2020) Pretreatment and process optimization of spent seaweed biomass (SSB) for bioethanol production using yeast (Saccharomyces cerevisiae). Renew Energy 153:456–471
Han W, Liu Y, Xu X, Huang J, He H, Chen L, Qiu S, Tang J, Hou P (2020) Bioethanol production from waste hamburger by enzymatic hydrolysis and fermentation. J Clean Prod 264:121658
Emiroğlu AO, Şen M (2018) Combustion, performance and emission characteristics of various alcohol blends in a single cylinder diesel engine. Fuel 212:34–40
Dhande DY, Sinaga N, Dahe KB (2021) Study on combustion, performance and exhaust emissions of bioethanol-gasoline blended spark ignition engine. Heliyon 7:e06380
Betiku E, Taiwo AE (2015) Modeling and optimization of bioethanol production from breadfruit starch hydrolyzate vis-à-vis response surface methodology and artificial neural network. Renew Energy 74:87–94
Manmai N, Unpaprom Y, Ramaraj R (2020) Bioethanol production from sunflower stalk: application of chemical and biological pretreatments by response surface methodology (RSM). Biomass Convers Biorefinery 3:1–15
Thongdumyu P, Intrasungkha N, Sompong O (2014) Optimization of ethanol production from food waste hydrolysate by co-culture of Zymomonas mobilis and Candida shehatae under non-sterile condition. African J Biotechnol 13:866–873
Castillo FJ, Izaguirre ME, Michelena V, Moreno B (1982) Optimization of fermentation conditions for ethanol production from whey. Biotechnol Lett 4:567–572
Chouaibi M, Ben Daoued K, Riguane K, Rouissi T, Ferrari G (2020) Production of bioethanol from pumpkin peel wastes: comparison between response surface methodology (RSM) and artificial neural networks (ANN). Ind Crops Prod 155:112822
Rudke AR, de Andrade CJ, Ferreira SRS (2020) Kappaphycus alvarezii macroalgae: an unexplored and valuable biomass for green biorefinery conversion. Trends Food Sci Technol 103:214–224
Sharma P, Gaur VK, Sirohi R, Varjani S, Kim SH, Wong JWC (2021) Sustainable processing of food waste for production of bio-based products for circular bioeconomy. Bioresour Technol 325:124684
Tun MM, Juchelková D, Tun MM, Juchelková D (2018) Drying methods for municipal solid waste quality improvement in the developed and developing countries: a review. Environ Eng Res 24:529–542
Markou G, Angelidaki I, Nerantzis E, Georgakakis D (2013) Bioethanol production by carbohydrate-enriched biomass of Arthrospira (Spirulina) platensis. Energies 6:3937–3950
Chala B, Oechsner H, Müller J (2019) Introducing temperature as variable parameter into kinetic models for anaerobic fermentation of coffee husk, pulp and mucilage. Appl Sci 9:412
Samsudin MDM, Don MM, Ibrahim N, Kasmani RM, Zakaria Z, Kamarudin KS (2017) Batch fermentation of bioethanol from the residues of Elaeis guineensis: optimisation using response surface methodology. Chem Eng Trans 56:1579–1584
Seenuvasan M, Sanjayini SJ, Kumar MA, Vinodhini G, Hellen Sathya J, Kumar VV (2017) Cellulase-mediated saccharification of lignocellulosic-rich pseudostem of Musa cavendish for bio-ethanol production by Saccharomyces cerevisiae MTCC 4779. Energy Sources Part A Recover Util Environ Eff 39:570–575
Ahmad N, Sahrin N, Talib N, Ghani FSA (2019) Characterization of energy content in food waste by using thermogravimetric analyser (TGA) and elemental analyser (CHNS-O). In: J Phys Conf Ser, IOP Publishing, 1349:12140
Ye N, Li D, Chen L, Zhang X, Xu D (2010) Comparative studies of the pyrolytic and kinetic characteristics of maize straw and the seaweed Ulva pertusa. PLoS One 5:e12641
Amuzu-Sefordzi B, Huang J, Gong M (2014) Hydrogen production by supercritical water gasification of food waste using nickel and alkali catalysts. WIT Trans Ecol Environ 190:285–296
Tesfaw A, Assefa F (2014) Current trends in bioethanol production by Saccharomyces cerevisiae: substrate, inhibitor reduction, growth variables, coculture, and immobilization. Int Sch Res Not 2014:1–11
Boudjema K, Fazouane-Naimi F, Hellal A (2015) Optimization of the bioethanol production on sweet cheese whey by Saccharomyces cerevisiae DIV13-Z087C0VS using response surface methodology (RSM). Rom Biotech Lett 20:10814–10825
Sharma N, Kalra KL, Oberoi HS, Bansal S (2007) Optimization of fermentation parameters for production of ethanol from kinnow waste and banana peels by simultaneous saccharification and fermentation. Indian J Microbiol 47:310–316
Alfenore S, Cameleyre X, Benbadis L, Bideaux C, Uribelarrea J-L, Goma G, Molina-Jouve C, Guillouet SE (2004) Aeration strategy: a need for very high ethanol performance in Saccharomyces cerevisiae fed-batch process. Appl Microbiol Biotechnol 63:537–542
de Albuquerque Wanderley AC, Soares ML, Gouveia ER (2014) Selection of inoculum size and Saccharomyces cerevisiae strain for ethanol production in simultaneous saccharification and fermentation (SSF) of sugar cane bagasse. Afr J Biotechnol 13:2762–2765
Sewsynker-Sukai Y, Kana EBG (2018) Simultaneous saccharification and bioethanol production from corn cobs: process optimization and kinetic studies. Bioresour Technol 262:32–41
Phukoetphim N, Salakkam A, Laopaiboon P, Laopaiboon L (2017) Kinetic models for batch ethanol production from sweet sorghum juice under normal and high gravity fermentations: logistic and modified Gompertz models. J Biotechnol 243:69–75
Chohan NA, Aruwajoye GS, Sewsynker-Sukai Y, Kana EBG (2020) Valorisation of potato peel wastes for bioethanol production using simultaneous saccharification and fermentation: process optimization and kinetic assessment. Renew Energy 146:1031–1040
Sebayang AH, Masjuki HH, Ong HC, Dharma S, Silitonga AS, Kusumo F, Milano J (2017) Optimization of bioethanol production from sorghum grains using artificial neural networks integrated with ant colony. Ind Crops Prod 97:146–155
Moodley P, Kana EBG (2019) Bioethanol production from sugarcane leaf waste: effect of various optimized pretreatments and fermentation conditions on process kinetics. Biotechnol Rep 22:e00329
Tarangini K, Kumar A, Satpathy GR, Sangal VK (2009) Statistical optimization of process parameters for Cr (VI) biosorption onto mixed cultures of Pseudomonas aeruginosa and Bacillus subtilis. Clean-Soil, Air, Water 37:319–327
Feng Y, Cai Z, Li H, Du Z, Liu X (2013) Response surface optimization of fluidized roasting reduction of low-grade pyrolusite coupling with pretreatment of stone coal. J Min Metall B Metall 49:33–41
Jayaprabakar J, Dawn SS, Ranjan A, Priyadharsini P, George RJ, Sadaf S, Rajha CR (2019) Process optimization for biodiesel production from sheep skin and its performance, emission and combustion characterization in CI engine. Energy 174:54–68
El-Gendy NS, Madian HR, Amr SSA (2013) Design and optimization of a process for sugarcane molasses fermentation by Saccharomyces cerevisiae using response surface methodology. Int J Microbiol 2013:1–9
Baş D, Boyacı IH (2007) Modeling and optimization I: Usability of response surface methodology. J Food Eng 78:836–845
Tiscione NB, Alford I, Yeatman DT, Shan X (2011) Ethanol analysis by headspace gas chromatography with simultaneous flame-ionization and mass spectrometry detection. J Anal Toxicol 35:501–511
Acknowledgements
The authors thank the Department of Chemistry, Sathyabama Institute of Science and Technology, for using rotary evaporator (SA-RE29T43, SPAN) specialty.
Funding
The authors thank the Ministry of Human Resource and Development (MHRD, Govt. Of India) Grant No: 5–5/2014-TS. VII 4th September 2014 for financial support in establishing the Centre of Excellence for Energy Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Priyadharsini, P., Dawn, S.S. Optimization of fermentation conditions using response surface methodology (RSM) with kinetic studies for the production of bioethanol from rejects of Kappaphycus alvarezii and solid food waste. Biomass Conv. Bioref. 13, 9977–9995 (2023). https://doi.org/10.1007/s13399-021-01819-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01819-w