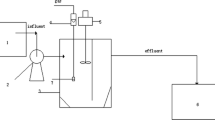
Abstract
Two-phase partitioning bioreactor (TPPB) model is favoured based on the use of a biocompatible, immiscible organic solvent in which high concentrations of pesticides are dissolved at normal temperature and pressure (NTP). This compound partitions into the aqueous phase containing microbial cells due to the metabolic activities of the cells and the interfacial tension generated between the two phases to weaken their energy barrier. Thus, the present study was carried out for bioremediation of chlorpyrifos in TPPB using bacterial consortia as an aqueous phase and hexadecane as an organic phase. The study showed that 93.44% chlorpyrifos was degraded by bacterial consortia within the time period of 15 days, creating a higher wetting spontaneity with − 10.3833 kJ/mol/K Gibb’s free energy with a higher surface area. The two-phase systems could be useful to salt-out or salt-in the specific pollutant across the phases to maintain the higher surface area for an efficient degradation.




















Similar content being viewed by others
References
Maya K, Singh RS, Upadhyay SN, Dubey SK (2011) Kinetic analysis reveals bacterial efficacy for biodegradation of chlorpyrifos and its hydrolyzing metabolite TCPS. Process Biochem 46:2130–2136
Singh BK, Walker A (2006) Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev 30:428–471
USEPA (1984) Analytical reference standards and supplemental data: the pesticides and industrial chemicals repository. USEPA Environmental Monitoring Systems Laboratory, Las Vegas
Bhatt P, Zhou X, Huang Y, Zhang W, Chen S (2021) Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. J Hazard Mater 411:125026. https://doi.org/10.1016/j.jhazmat.2020.125026
Singh BK, Walker A, Wright DJ (2006) Bioremedial potential of fenamiphos and chlorpyrifos degrading isolates: influence of different environmental conditions. Soil Biol Biochem 38:2682–2693
Kulshretha G, Kumari A (2011) Fungal degradation of chlorpyrifos by Acremonium sp. strain (GFRC-1) isolated from a laboratory-enriched red agricultural soil. Biol Fert Soils 47:219–225
Chowdhury A, Pradhan S, Saha M, Sanyal N (2008) Impact of pesticides on soil microbiological parameters and possible bioremediation strategies. Ind J Microbiol 48:114–127
Huang Y, Zhang W, Pang S, Chen J, Bhatt P, Mishra S, Chen S (2021) Insights into the microbial degradation and catalytic mechanisms of chlorpyrifos. Environ Res 194:110660. https://doi.org/10.1016/j.envres.2020.110660
Dhanya MS (2014) Advances in microbial biodegradation of chlorpyrifos. J Environ Res Dev 9:232–240
Bhatt P, Bhatt K, Sharma A, Zhang W, Mishra S, Chen S (2021) Biotechnological basis of microbial consortia for the removal of pesticides from the environment. Crit Rev Biotechnol 41(3):317–338. https://doi.org/10.1080/07388551.2020.1853032
Fulekar MH, Geetha M (2008) Bioremediation of Chlorpyrifos by Pseudomonas aeruginosa using scale up technique. J Appl Biosci 12:657–660
Bhatt P, Gangola S, Bhandari G, Zhang W, Maithani D, Mishra S, Chen S (2021) New insights into the degradation of synthetic pollutants in contaminated environments. Chemosphere 268:128827. https://doi.org/10.1016/j.chemosphere.2020.128827
Singh M, Sushma, (2009) Studies of densities, apparent molal volume, viscosities, surface tension and free energies of activation for interactions of praseodymium Sal2en complex with dimethylsulphoxide. J Mol Liq 148:6–12
Singh M (2008) Survismeter 3-in-1 for interfacial tension (IFT), surface tension and viscosity measurements simultaneously. Surf Interface Anal 40(2):76–80
Singh M, Kumar V (2008) Solvodynamics of benzene and water phases by DTAB, MTOAC, TMSOI and orcinol studied with interfacial tension, surface tension and viscosity measured with survismeter. Int J Thermodyn 11:181–186
Hong QH, Li DT, Hao CY (2005) Identification of soybean varieties by direct determination of FTIR spectrum. Spectrosc Spectr Anal 8:1246–1249
Van FR, Sedman J, Yaylayan V (2004) Quantitative determination of moisture in lubricants by Fourier transform infrared spectroscopy. Appl Spectrosc 2:193–198
Adams MJ, Romeo MJ, Rawson P (2007) FTIR analysis and monitoring of synthetic aviation engine oils. Talanta 4:629–634
Cailteau C, Pironon J, de Donato P (2011) FTIR metrology aspects for on-line monitoring of CO2 and CH4 in underground laboratory conditions. Anal Methods 4:877–887
Esler MB, Griffith DWT, Wilson SR (2000) Precision trace gas analysis by FTIR spectroscopy. 1. Simultaneous analysis of CO2, CH4, N2O, and CO in air. Anal Chem 1:206–215
Chen S, Liu C, Peng C, Liu H, Hu M et al (2012) Biodegradation of chlorpyrifos and its hydrolysis product 3,5,6-trichloro-2-pyridinol by a new fungal strain Cladosporium cladosporioides Hu-01. PLoS ONE 7(10):e47205. https://doi.org/10.1371/journal.pone.0047205
Sasikala C, Jiwal S, Rout P et al (2012) Biodegradation of chlorpyrifos by bacterial consortium isolated from agriculture soil. World J Microbiol Biotechnol 28:1301–1308. https://doi.org/10.1007/s11274-011-0879-z
Uniyal S, Sharma RK, Kondakal V (2021) New insights into the biodegradation of chlorpyrifos by a novel bacterial consortium: Process optimization using general factorial experimental design. Ecotoxicol Environ Saf 209:111799. https://doi.org/10.1016/j.ecoenv.2020.111799
Sun X, Chen L, Liu C, Yin Xu, Ma W, Ni H (2020) Biodegradation of CP/TCP by a constructed microbial consortium after comparative bacterial community analysis of long-term CP domesticated activated sludge. J Environ Sci Health B 55(10):898–908. https://doi.org/10.1080/03601234.2020.1794453
Farhan M, Ahmad M, Kanwal A et al (2021) Biodegradation of chlorpyrifos using isolates from contaminated agricultural soil, its kinetic studies. Sci Rep 11:10320. https://doi.org/10.1038/s41598-021-88264-x
Silambarasan S, Abraham J (2013) Ecofriendly method for bioremediation of Chlorpyrifos from agricultural soil by novel fungus Aspergillus terreus JAS1. Water Air Soil Pollut 224:1369. https://doi.org/10.1007/s11270-012-1369-0
Neti N, Zakkula V (2013) Analysis of chlorpyrifos degradation by Kocuria sp. using GC and FTIR. Current Biotica 6:466–472
Siddique T, Okeke BC, Arshad M, Frankenberger WT (2003) Biodegradation kinetics of endosulfan by Fusarium ventricosum and a Pandoraea species. J Agric Food Chem 51:8015–8019
Geetha M, Fulekar MH (2008) Bioremediation of pesticides in surface soil treatment unit using microbial consortia. Afr J Environ Sci Technol 2(2):036–045
Acknowledgements
We acknowledge the Central University of Gujarat, Gandhinagar, to fulfil the required facilities for the research work. The authors also wish to thank the Gujarat State Biotechnology Mission (GSBTM), Gandhinagar, for 16S rRNA sequencing facilities for identification of bacteria.
Funding
RKR was supported by fellowships from the University Grant Commission, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Significant statement
The two-phase systems were found effective for bioremediation of chlorpyrifos using cow dung bacterial consortia as novel source of biomass. The finding shows unique study, i.e., determination of interracial tension and Gibbs free energy that illustrated the bioremediation of chlorpyrifos in two-phase partitioning bioreactor. The system is able to degrade high concentration of chlorpyrifos, and thus can be used for environmental clean-up approaches.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ravi, R.K., Singh, M. & Fulekar, M.H. Bioconversion dynamics for bioremediation of chlorpyrifos pesticide using bacterial consortia in two immiscible liquid phase partitioning bioreactor. Biomass Conv. Bioref. 12, 1755–1769 (2022). https://doi.org/10.1007/s13399-021-01695-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01695-4