Abstract
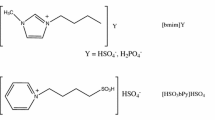
Triethylammonium hydrogen sulfate (TEAHS) has been employed as an inexpensive protic ionic liquid catalyst for the preparation of various biomass-derived renewable compounds. TEAHS efficiently catalyzed the esterification of biomass-derived chemical intermediates such as levulinic acid, 2-furoic acid, stearic acid, and isosorbide. The scalable, cosolvent-free preparations were conducted in a batch-type glass pressure reactor, which provided excellent yields (> 80%) of the esters under moderate conditions. The TEAHS catalyst was conveniently separated from the reaction mixture and reused without significant loss of activity.
Graphical abstract







Similar content being viewed by others
References
Demirbas A (2001) Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers Manag 42:1357–1378. https://doi.org/10.1016/S0196-8904(00)00137-0
Jing Y, Guo Y, Xia Q, Liu X, Wang Y (2019) Catalytic production of value-added chemicals and liquid fuels from lignocellulosic biomass. Chem. 5:2520–2546. https://doi.org/10.1016/j.chempr.2019.05.022
Sudarsanam P, Zhong R, Van den Bosch S, Coman SM, Parvulescu VI, Sels BF (2018) Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem Soc Rev 47:8349–8402. https://doi.org/10.1039/C8CS00410B
De S, Dutta S, Saha B (2016) Critical design of heterogeneous catalysts for biomass valorization: current thrust and emerging prospects. Catal Sci Technol 6:7364–7385. https://doi.org/10.1039/C6CY01370H
Hara M, Nakajima K, Kamata K (2015) Recent progress in the development of solid catalysts for biomass conversion into high value-added chemicals. Sci Technol Adv Mater 16:034903. https://doi.org/10.1088/1468-6996/16/3/034903
Fang D, Zhou XL, Ye Z-W, Liu Z-L (2006) Brønsted acidic ionic liquids and their use as dual solvent-catalysts for Fischer esterifications. Ind Eng Chem Res 45:7982–7984. https://doi.org/10.1021/ie060365d
Parvelescu VI, Hardacre C (2007) Catalysis in ionic liquids. Chem Rev 107:2615–2665. https://doi.org/10.1021/cr050948h
Karimi B, Tavakolian M, Akbari M, Mansouri F (2018) Ionic liquids in asymmetric synthesis: an overall view from reaction media to supported ionic liquid catalysis. ChemCatChem 10:3173–3205. https://doi.org/10.1002/cctc.201701919
Sheldon R (2001) Catalytic reactions in ionic liquids. Chem Commun 37:2399–2407. https://doi.org/10.1039/B107270F
Bano K, Jain A, Sarkar R, Panda TK (2020) Economically viable and efficient catalysts for esterification and cross aldol condensation reactions under mild conditions. Chemistryselect 5(15):4470–4477. https://doi.org/10.1002/slct.202000252
Troter DZ, Todorovid ZB, Dokic-Stojanovic DR, Stamenkovic OS, Veljkovic VB (2016) Application of ionic liquids and deep eutectic solvents in biodiesel production: a review. Renew Sust Energ Rev 61:473–500. https://doi.org/10.1016/j.rser.2016.04.011
Liu C-Z, Wang F, Stiles AR, Guo C (2012) Ionic liquids for biofuel production: opportunities and challenges. Appl Energy 92:406–414. https://doi.org/10.1016/j.apenergy.2011.11.031
Tiong YW, Yap CL, Gan S, Yap WSP (2018) Conversion of biomass and its derivatives to levulinic acid and levulinate esters via ionic liquids. Ind Eng Chem Res 57:4749–4766. https://doi.org/10.1021/acs.iecr.8b00273
Kumar K, Dahiya A, Patra T, Upadhyayula S (2018) Upgrading of HMF and biomass-derived acids into HMF esters using bifunctional ionic liquid catalysts under solvent free conditions. ChemistrySelect 3:6242–6248. https://doi.org/10.1002/slct.201800903
Kraus GA, Guney T (2012) A direct synthesis of 5-alkoxymethylfurfural ethers from fructose via sulfonic acid-functionalized ionic liquids. Green Chem 14:1593–1596. https://doi.org/10.1039/C2GC35175G
Chidambaram M, Bell AT (2010) A two-step approach for the catalytic conversion of glucose to 2, 5-dimethylfuran in ionic liquids. Green Chem 12:1253–1262. https://doi.org/10.1039/C004343E
Vekariya RL (2017) A review of ionic liquids: applications towards catalytic organic transformations. J Mol Liq 227:44–60. https://doi.org/10.1016/j.molliq.2016.11.123
Chen L, Sharifzadeh M, Dowell NM, Welton T, Shah N, Hallett JP (2014) Inexpensive ionic liquids:[HSO4]-based solvent production at bulk scale. Green Chem 16:3098–3106. https://doi.org/10.1039/C4GC00016A
Ganeshpure PA, George G, Das J (2007) Application of triethylammonium salts as ionic liquid catalyst and medium for Fischer esterification. Arkivoc 8:273–278. https://doi.org/10.3998/ark.5550190.0008.821
Karimi-Jaberi Z, Masoudi B, Rahmani A, Alborzi K (2017) Triethylammonium hydrogen sulfate [Et3NH] [HSO4] as an efficient ionic liquid catalyst for the synthesis of coumarin derivatives. Polycyclic Aromat Compd 40:99–107. https://doi.org/10.1080/10406638.2017.1363061
Siddiqui ZN, Khan K (2014) [Et3NH][HSO4]-catalyzed efficient, eco-friendly, and sustainable synthesis of quinoline derivatives via Knoevenagel condensation. ACS Sustain Chem Eng 2:1187–1194. https://doi.org/10.1021/sc500023q
Nimbalkar UD, Seijas JA, Vazquez-Tato MP, Damale MG, Sangshetti JN, Nikalje APG (2017) Ionic liquid-catalyzed green protocol for multi-component synthesis of dihydropyrano [2,3-c] pyrazoles as potential anticancer scaffolds. Molecules 22:1628. https://doi.org/10.3390/molecules22101628
Dastyar W, Zhao M, Yuan W, Li H, Ting ZJ, Ghaedi H, Yuan H, Li X, Wang W (2019) Effective pretreatment of heavy metal-contaminated biomass using a low-cost ionic liquid (triethylammonium hydrogen sulfate): optimization by response surface methodology–box Behnken design. ACS Sustain Chem Eng 7:11571–11581. https://doi.org/10.1021/acssuschemeng.9b01457
Gschwend FJV, Malaret F, Shinde S, Brandt-Talbot A, Hallett JP (2018) Rapid pretreatment of Miscanthus using the low-cost ionic liquid triethylammonium hydrogen sulfate at elevated temperatures. Green Chem 20:3486–3498. https://doi.org/10.1039/C8GC00837J
Brandt-Talbot A, Gschwend FJV, Fennell PS, Lammens TM, Tan B, Weale J, Hallett JP (2017) An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem 19:3078–3102. https://doi.org/10.1039/C7GC00705A
Man Z, Elsheikh YA, Bustam MA, Yusup S, Mutalib MIA, Muhammad N (2013) A Brønsted ammonium ionic liquid-KOH two-stage catalyst for biodiesel synthesis from crude palm oil. Ind Crop Prod 41:144–149. https://doi.org/10.1016/j.indcrop.2012.04.032
Demolis A, Essayem N, Rataboul F (2014) Synthesis and applications of alkyl levulinates. ACS Sustain Chem Eng 2:1338–1352. https://doi.org/10.1021/sc500082n
Yan L, Yao Q, Fu Y (2017) Conversion of levulinic acid and alkyl levulinates into biofuels and high-value chemicals. Green Chem 19:5527–5547. https://doi.org/10.1039/C7GC02503C
Usha HS, Maitra S (2016) Synthesis characterization and application of polyglycerol esters of fatty acids: biodegradable surfactants. J Dispers Sci Technol 37:41–47. https://doi.org/10.1080/01932691.2015.1025137
Mehta B, Kathalewar M, Sabnis A (2014) Diester based on castor oil fatty acid as plasticizer for poly (vinyl chloride). J Appl Polym Sci 131:40354. https://doi.org/10.1002/app.40354
Veillette M, Fendler AG, Faucheux N, Heitz M (2017) Esterification of free fatty acids with methanol to biodiesel using heterogeneous catalysts: from model acid oil to microalgae lipids. Chem Eng J 308:101–109. https://doi.org/10.1016/j.cej.2016.07.061
Vieira SS, Magriotis ZM, Santos NAV, Saczk AA, Hori CE, Arroyo PA (2013) Biodiesel production by free fatty acid esterification using lanthanum (La3+) and HZSM-5 based catalysts. Bioresour Technol 133:248–255. https://doi.org/10.1016/j.biortech.2013.01.107
Manzoli M, Menegazzo F, Signoretto M, Marchese D (2016) Biomass derived chemicals: furfural oxidative esterification to methyl-2-furoate over gold catalysts. Catalysts 6:107. https://doi.org/10.3390/catal6070107
Dussenne C, Delaunay T, Wiatz V, Wyart H, Suisse I, Sauthier M (2017) Synthesis of isosorbide: an overview of challenging reactions. Green Chem 19:5332-5344. https://doi.org/10.1039/C7GC01912B
Wilson WC (1926) 2-Furancarboxylic acid and 2-furylcarbinol. Org Synth 6:44. https://doi.org/10.15227/orgsyn.006.0044
Acknowledgments
The authors want to thank TIFR, Hyderabad for collecting NMR (1H and 13C) samples.
Funding
This study is financially supported by Science and Engineering Research Board (SERB), India, under the grant number YSS/2015/001649 and Vision Group of Science and Technology (VGST) Project No. KSTePS/VGST-RGS-F/2018-19/GRD No. 806/315.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
The details of the spectroscopic characterization (FTIR, 1H-NMR, 13C-NMR) of the synthesized compounds, and the TEAHS catalyst recovery are provided as supporting information. (PDF 1.89 mb)
Rights and permissions
About this article
Cite this article
Bhat, N.S., Mal, S.S. & Dutta, S. [Et3NH][HSO4] as an efficient and inexpensive ionic liquid catalyst for the scalable preparation of biorenewable chemicals. Biomass Conv. Bioref. 12, 5619–5625 (2022). https://doi.org/10.1007/s13399-020-01052-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01052-x