Abstract
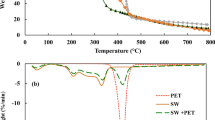
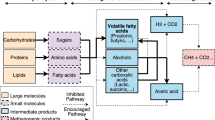
The hydrothermal liquefaction (HTL) process is a promising direction for the conversion of waste biomass into valuable chemicals/fuels. Optimization of the process parameters for HTL of biomass has been the research scope in recent years. The study aims to examine the effects of process variables such as the effect of temperature, the role of solvents: water (H2O), methanol (CH3OH), and ethanol (C2H5OH) on hydrothermal liquefaction of the corncob biomass. Hydrothermal liquefaction has been carried out at temperatures of 260, 280, and 300 °C with a reaction holding time of 15 min. The maximum bio-oil yield was obtained (38.0 wt%) at 280 °C in the presence of H2O, whereas in the case of CH3OH and C2H5OH solvents, the maximum bio-oil yield was 18.67 wt.% and 16.83 wt.% at 260 °C and 300 °C, respectively. The solvent efficiency in corncob liquefaction was observed as H2O ˃ CH3OH ˃ C2H5OH for bio-oil production. The obtained bio-oils were characterized in detail using GC-MS, FT-IR, and 1H NMR. From the GC-MS analysis of bio-oil, the phenolic compounds were observed to a higher degree (60%) in the case of H2O solvent than CH3OH and C2H5OH solvent (57% and 46%). Also, FT-IR analysis showed that higher aromatic functional groups present in bio-oil obtained with water solvent while liquefaction with alcoholic solvent showed higher ester functional groups. Moreover, the analysis of bio-residues indicated that the decomposition of the corncob occurred in a different way during HTL with the presence of different solvents.






Similar content being viewed by others
References
Chena WH, Liu SH, Juang TT, Tsai CM, Zhuanga YQ (2015) Characterization of solid and liquid products from bamboo torrefaction. Appl Energy 160:829–835
Gan J, Yuan W, Nelson NO, Agudelo SC (2010) Hydrothermal conversion of Corncobs and crude glycerol. Biol Eng Trans 2:197–210
Zhang Q, Hu JJ, Lee DJ (2017) Pretreatment of biomass using ionic liquids: research updates. Renew Energy 111:77–84
Guo H, Chang Y, Lee DJ (2018) Enzymatic saccharification of lignocellulosic biorefinery: Research focuses 252:198-215
Hardi F, Mäkelä M, Yoshikawa K (2017) Non-catalytic hydrothermal liquefaction of pine sawdust using experimental design: material balances and products analysis. Appl Energy 204:1026–1034
Biswas B, Kumar AA, Bisht Y, Singh R, Kumar J, Bhaskar T (2017) Effects of temperature and solvent on hydrothermal liquefaction of Sargassumtenerrimum algae. Bioresour Technol 242:344–350
Liu HM, Li MF, Yang S, Sun RC (2013) Understanding the mechanism of cypress liquefaction in hot-compressed water through characterization of solid residues. Energies 6:1590–1603
Ramirez JA, Brown R, Rainey TJ (2018) Techno-economic analysis of the thermal liquefaction of sugarcane bagasse in ethanol to produce liquid fuels. Appl Energy 224:184–193
Khampuang K, Boreriboon N, Prasassarakich P (2015) Alkali catalyzed liquefaction of corncob in supercritical ethanol water. Biomass Bioenergy 85:460–466
Demirbas A (2008) Conversion of corn stover to chemicals and fuels. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 30:788–796
Zhou D, Zhang S, Fu H, Chen J (2012) Liquefaction of macroalgae Enteromorphaprolifera in sub-/supercritical alcohol: direct production of ester compounds. Energy Fuel 26:2342–2351
Liu Z, Zhang FS (2008) Effects of various solvents on the liquefaction of biomass to produce fuels and chemical feedstocks. Energy Convers Manag 49:3498–3504
Gan J, Yuan W (2013) Operating condition optimization of corncob hydrothermal conversion for bio-oil production. Appl Energy 103:350–357
Durak H (2019) Characterization of products obtained from hydrothermal liquefaction of biomass (Anchusa azurea) compared to other thermochemical conversion methods. Biomass Conversion Biorefinery 9:459–470
Singh R, Balagurumurthy B, Bhaskar T (2015) Hydrothermal liquefaction of macro algae: effect of feedstock Composition. Fuel 146:69–74
Yu J, Paterson N, Blamey J, Millan M (2017) Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 191:140–149
Biswas B, Krishna BB, Singh R, Kumar J, Bhaskar T (2016) Slow pyrolysis of pine wood: effect of CO2 and N2 atmosphere. J Energy Environ Sustain 2:7–12
Li R, Li B, Yang T, Xie Y, Kai X (2014) Production of bio-oil from rice stalk supercritical ethanol liquefaction combined with the torrefaction process. Energy Fuel 28:1948–1955
Via BK, Adhikari S, Taylor S (2013) Modeling for proximate analysis and heating value of torrefied biomass with vibration spectroscopy. Bioresour Technol 133:1–8
Zhang Z, Zhu M, Zhang DA (2018) Thermogravimetric study of the characteristics of pyrolysis of cellulose isolated from selected biomass. Appl Energy 220:87–93
Yan HL, Zong ZM, Zhu W-W, Li ZK, Wang YG, Wei ZH, Li Y, Wei XY (2015) Poplar liquefaction in water/methanol co-solvents. Energy Fuel 29:3104–3110
Chen Y, Wu Y, Hua D, Li C, Harold MP, Wang J, Yang M (2015) Thermochemical conversion of low-lipid microalgae for the production of liquid fuels: challenges and opportunities. RSC Adv 5:18673–18701
Yang C, Jia L, Chen C, Liu G, Fang W (2011) Bio-crude oil from hydro-liquefaction of Dunaliella salina over Ni/REHY catalyst. Bioresour Technol 102:4580–4584
Wang Y, Wang H, Lin H, Zheng Y, Zhao J, Pelletier A, Li K (2013) Effects of solvents and catalysts in liquefaction of pinewood sawdust for the production of biocrude oils. Biomass Bioenergy 59:158–167
Mullen CA, Strahan GD, Boateng AA (2009) Characterization of various fast-pyrolysisbio-oils by NMR spectroscopy. Energy Fuel 23:2707–2718
Kosa M, Ben H, Theliander H, Ragauskas AJ (2011) Pyrolysis oils from CO2 precipitated Kraft lignin. Green Chem 13:3196–3202
Biswas B, Singh R, Kumar J, Khan AA, Krishna BB, Bhaskar T (2016) Slow pyrolysis of prot, alkali and dealkaline lignins for production of chemicals, Bioresour. Technol 213:319–326
Cao L, Zhang C, Chen H, Tsang DCW, Luo G, Zhang S, Chen J (2017) Hydrothermal liquefaction of agricultural and forestry wastes: state-of-the- art review and future prospects. Bioresour Technol 245:1184–1193
Gollakota ARK, Kishore N, Gu S (2018) A review on hydrothermal liquefaction of biomass. Renew Sust Energ Rev 81:1378–1392
Liua H, Ma M, Xie X (2017) New materials from solid residues for investigation the mechanism of biomass hydrothermal liquefaction. Ind Crop Prod 108:63–71
Barbier J, Charon N, Dupassieux N, Loppinet-Serani A, Mahé L, Ponthus J, Courtiade M, Ducrozet A, Quoineaud AA, Cansell F (2012) Hydrothermal conversion of lignin compounds. A detailed study of fragmentation and condensation reaction pathways. Biomass Bioenergy 46:479–491
Kang SM, Li XL, Fan J, Chang J (2011) Classified separation of lignin hydrothermal liquefied products. Ind Eng Chem Res 50:11288–11296
Roberts VM, Stein V, Reiner T, Lemonidou A, Li X, Lercher JA (2011) Towards quantitative catalytic lignin depolymerization. Chem Eur J 17:5939–5948
Song Q, Wang F, Cai J, Wang Y, Zhang J, Yu W, Xu J (2013) Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ Sci 6:994–1007
Pińkowska H, Wolak P, Złocińska A (2011) Hydrothermal decomposition of xylan as a model substance for plant biomass waste-hydrothermolysis in subcritical water. Biomass Bioenergy 35:3902–3912
Lee J, Yang X, Choa SH, Kim JK, Lee SS, Tsang DCW, Ok YS, Kwon EE (2017) Pyrolysis process of agricultural waste using CO2 for waste management, energy recovery, and biochar fabrication. Appl Energy 185:214–222
Uchimiya M, Orlov A, Ramakrishnan G, Sistani K (2013) In situ and ex situ spectroscopic monitoring of bio-char’s surface functional groups. J Anal Appl Pyrolysis 102:53–59
Wang Z, Coa J, Wang J (2009) Pyrolitic characteristics of pine wood in a slowly heating and gas sweeping fixed-bed ractor. J Anal Appl Pyrolysis 84:179–184
Shaabana A, Sea SM, Mitan NMM, Dimina MF (2013) Characterization of biochar derived from rubber wood sawdust through slow pyrolysis on surface porosities and functional groups. Proc Eng 68:365–371
Usman ARA, Abduljabbar A, Vithanage M, Ok YS, Ahmad M, Ahmad M, Elfaki J, Abdulazeem SS, Al-Wabel MI (2015) Biochar production from date palm waste: charring temperature induced changes in composition and surface chemistry. J Anal Appl Pyrolysis 115:392–400
Acknowledgment
The authors thank the Director, CSIR-Indian Institute of Petroleum, Dehradun, for his constant encouragement and support and AcSIR for granting permission to conduct this research work at CSIR-IIP. Bijoy Biswas thanks CSIR, New Delhi, India, for his Senior Research Fellowship (SRF). The authors thank the Analytical Science Division (ASD) of CSIR-IIP for NMR, FT-IR, and XRD analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary materials
ESM 1
(DOCX 134 kb)
Rights and permissions
About this article
Cite this article
Biswas, B., Bisht, Y., Kumar, J. et al. Effects of temperature and solvent on hydrothermal liquefaction of the corncob for production of phenolic monomers. Biomass Conv. Bioref. 12, 91–101 (2022). https://doi.org/10.1007/s13399-020-01012-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01012-5