Abstract
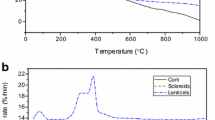
Pyrolysis kinetics of Quercus cerris cork was investigated using thermogravimetric analysis with heating rates of 10, 20, 50, and 100 °C min−1. The activation energies and chemical compositions of cork components were determined by different model-fitting methods, isoconversional Kissinger-Akahira-Sunose (KAS) method, and Lorentzian multi-peak fitting. Wet chemical analysis of cork was conducted to compare with the chemical compositions predicted by the kinetic models and Lorentzian multi-peak fitting. The results show that pyrolysis of Quercus cerris cork possibly follows nth-order kinetics, and best fits to the experimental data were obtained by three-halves kinetics followed by first-order and contracting sphere models. The fit qualities of the different models were close implying that the first order models could be used for practical applications. Six pseudo-components approximation used in these models suggested that while cork hemicelluloses and cellulose undergo thermal decompositions similar as in wood, cork suberin decomposes in two distinct steps, i.e., cellulose-like and lignin-like decompositions. Isoconversional KAS method showed that the average activation energy of Quercus cerris cork is approximately 298 kJ mol−1. The reconstructed mass loss curves after Lorentzian multi-peak fitting resulted in smaller activation energies for cork components.









Similar content being viewed by others
Abbreviations
- Activation energy:
-
Ea J mol−1
- Universal gas constant:
-
R 8.314 J mol−1 K−1
- Heating rate:
-
HR, β °C min−1
- Temperature:
-
T K, °C
- Time:
-
t min
- Central peak temperature:
-
Tc °C
- Peak width:
-
w °C
- Peak area:
-
A 0.5 °C (% min−1)
- Heat transfer coefficient:
-
h W m−2 K−1
- Thermal conductivity:
-
k W m−1 K−1
- Thermogravimetric analysis:
-
TGA
- Differential thermogravimetry:
-
DTG
- Conversion degree:
-
α
- Pre-exponential factor:
-
k 0 , A α
- Biomass fraction:
-
xi
- Residual sum of squares:
-
RSS
- Lorentzian multi-peak fitting:
-
LMPF
- Kissinger-Akahira-Sunose:
-
KAS
References
Pereira H (2007) Cork: biology, production and uses
Şen A, Miranda I, Ferreira J et al (2018) Chemical composition and cellular structure of ponytail palm (Beaucarnea recurvata) cork. Ind Crop Prod 124:845–855
Şen A, Leite C, Lima L, Lopes P, Pereira H (2016) Industrial valorization of Quercus cerris bark: pilot scale fractionation. Ind Crop Prod 92:42–49. https://doi.org/10.1016/j.indcrop.2016.07.044
Ingram L, Mohan D, Bricka M et al (2008) Pyrolysis of wood and bark in an auger reactor: physical properties and chemical analysis of the produced bio-oils. Energy Fuel 22:614–625
Mohan D, Pittman CU Jr, Bricka M et al (2007) Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J Colloid Interface Sci 310:57–73
Lievens C, Mourant D, Gunawan R, Hu X, Wang Y (2015) Organic compounds leached from fast pyrolysis mallee leaf and bark biochars. Chemosphere 139:659–664
Pereira H (1992) The thermochemical degradation of cork. Wood Sci Technol 26:259–269
Neto CP, Rocha J, Gil A, Cordeiro N, Esculcas AP, Rocha S, Delgadillo I, de Jesus JDP, Correia AJF (1995) 13C solid-state nuclear magnetic resonance and Fourier transform infrared studies of the thermal decomposition of cork. Solid State Nucl Magn Reson 4:143–151
Şen A, Van Den Bulcke J, Defoirdt N et al (2014) Thermal behaviour of cork and cork components. Thermochim Acta 582:94–100. https://doi.org/10.1016/j.tca.2014.03.007
Froment GF, Bischoff KB, De Wilde J (1990) Chemical reactor analysis and design. Wiley New York
Khawam A, Flanagan DR (2006) Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B 110:17315–17328
Cai J, Xu D, Dong Z, Yu X, Yang Y, Banks SW, Bridgwater AV (2018) Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: case study of corn stalk. Renew Sust Energ Rev 82:2705–2715
Sanchez-Silva L, López-González D, Villaseñor J, Sánchez P, Valverde JL (2012) Thermogravimetric–mass spectrometric analysis of lignocellulosic and marine biomass pyrolysis. Bioresour Technol 109:163–172
Khawam A, Flanagan DR (2005) Role of isoconversional methods in varying activation energies of solid-state kinetics: II. Nonisothermal kinetic studies. Thermochim Acta 436:101–112
Anca-Couce A, Berger A, Zobel N (2014) How to determine consistent biomass pyrolysis kinetics in a parallel reaction scheme. Fuel 123:230–240
Slopiecka K, Bartocci P, Fantozzi F (2012) Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl Energy 97:491–497
Orfão JJM, Antunes FJA, Figueiredo JL (1999) Pyrolysis kinetics of lignocellulosic materials—three independent reactions model. Fuel 78:349–358
Di Blasi C (2008) Modeling chemical and physical processes of wood and biomass pyrolysis. Prog Energy Combust Sci 34:47–90
Cordeiro N, Blayo A, Belgacem NM, Gandini A, Pascoal Neto C, LeNest JF (2000) Cork suberin as an additive in offset lithographic printing inks. Ind Crop Prod 11:63–71
Shangguan W, Chen Z, Zhao J, Song X (2018) Thermogravimetric analysis of cork and cork components from Quercus variabilis. Wood Sci Technol 52:181–192
Şen A, Miranda I, Santos S, Graça J, Pereira H (2010) The chemical composition of cork and phloem in the rhytidome of Quercus cerris bark. Ind Crop Prod 31:417–422. https://doi.org/10.1016/j.indcrop.2010.01.002
Ferreira JPA, Quilhó T, Pereira H (2017) Characterization of Betula pendula outer bark regarding cork and phloem components at chemical and structural levels in view of biorefinery integration. J Wood Chem Technol 37:10–25
Zhou Z, Han L, Bollas GM (2014) Kinetics of NiO reduction by H2 and Ni oxidation at conditions relevant to chemical-looping combustion and reforming. Int J Hydrog Energy 39:8535–8556
Dhyani V, Bhaskar T (2018) Kinetic analysis of biomass pyrolysis. In: Waste Biorefinery. Elsevier, pp 39–83
Xu Q, Zhang H, Li H, Zhao S, Wan L, Yan Y (2013) Pyrolysis kinetics mechanism analysis of sawdust by Sestak-Berggren function. Energy Sources, A Recov Util Environ Eff 35:936–944
Burnham AK, Braun RL (1999) Global kinetic analysis of complex materials. Energy Fuel 13:1–22
Hu S, Jess A, Xu M (2007) Kinetic study of Chinese biomass slow pyrolysis: comparison of different kinetic models. Fuel 86:2778–2788
Tran K-Q, Bach Q-V, Trinh TT, Seisenbaeva G (2014) Non-isothermal pyrolysis of torrefied stump–a comparative kinetic evaluation. Appl Energy 136:759–766
Vyazovkin S, Burnham AK, Criado JM et al (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19
Varhegyi G, Antal MJ Jr, Szekely T, Szabo P (1989) Kinetics of the thermal decomposition of cellulose, hemicellulose, and sugarcane bagasse. Energy Fuel 3:329–335
Bartocci P, Tschentscher R, Stensrød RE, Barbanera M, Fantozzi F (2019) Kinetic analysis of digestate slow pyrolysis with the application of the master-plots method and independent parallel reactions scheme. Molecules 24:1657
Schwaab M, Pinto JC (2007) Optimum reference temperature for reparameterization of the Arrhenius equation. Part 1: Problems involving one kinetic constant. Chem Eng Sci 62:2750–2764
Schwaab M, Lemos LP, Pinto JC (2008) Optimum reference temperature for reparameterization of the Arrhenius equation. Part 2: Problems involving multiple reparameterizations. Chem Eng Sci 63:2895–2906
Standl S, Hinrichsen O (2018) Kinetic modeling of catalytic olefin cracking and methanol-to-olefins (MTO) over zeolites: A review. Catalysts 8:626
Katsikas L, Popović IG (2003) Improvement to the Flynn−Wall method of determining apparent activation energies of the thermal degradation of polymers. J Phys Chem B 107:7522–7525
Wang Q, Jia C, Jiang Q, Wang Y, Wu D (2014) Pyrolysis model of oil sand using thermogravimetric analysis. J Therm Anal Calorim 116:499–509
Sen A, Zhianski M, Glushkova M, Petkova K, Ferreira J, Pereira H (2016) Chemical composition and cellular structure of corks from Quercus suber trees planted in Bulgaria and Turkey. Wood Sci Technol 50:1261–1276. https://doi.org/10.1007/s00226-016-0836-y
Ranzi E, Cuoci A, Faravelli T, Frassoldati A, Migliavacca G, Pierucci S, Sommariva S (2008) Chemical kinetics of biomass pyrolysis. Energy Fuel 22:4292–4300
Yuzay IE, Auras R, Soto-Valdez H, Selke S (2010) Effects of synthetic and natural zeolites on morphology and thermal degradation of poly (lactic acid) composites. Polym Degrad Stab 95:1769–1777
Wang T, Zhang R, Su W, Lu Q, Dong C (2016) Study on pyrolysis characteristics of red pepper stalks to analyze the changes of pyrolytic behaviors from xylophyta to herbage. J Anal Appl Pyrolysis 120:330–333
Demirbas A (2004) Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J Anal Appl Pyrolysis 72:243–248
Van de Velden M, Baeyens J, Brems A et al (2010) Fundamentals, kinetics and endothermicity of the biomass pyrolysis reaction. Renew Energy 35:232–242
Lakreb N, As N, Gorgun V, Sen U, Gomes MG, Pereira H (2018) Production and characterization of particleboards from cork-rich Quercus cerris bark. Eur J Wood Wood Prod 76:989–997. https://doi.org/10.1007/s00107-017-1284-6
Alvarez VA, Vázquez A (2004) Thermal degradation of cellulose derivatives/starch blends and sisal fibre biocomposites. Polym Degrad Stab 84:13–21
Yi Z, Li C, Jiang J, Zhang J, Zhang W, Li J (2015) Pyrolysis kinetics of tannin–phenol–formaldehyde resin by non-isothermal thermogravimetric analysis. J Therm Anal Calorim 121:867–876
Yaws CL, Gabbula C (2003) Yaws" Handbook of thermodynamic and physical properties of chemical compounds. Knovel
Patwardhan PR, Brown RC, Shanks BH (2011) Understanding the fast pyrolysis of lignin. ChemSusChem 4:1629–1636
Grønli MG, Várhegyi G, Di Blasi C (2002) Thermogravimetric analysis and devolatilization kinetics of wood. Ind Eng Chem Res 41:4201–4208
Park HJ, Dong J-I, Jeon J-K, Park YK, Yoo KS, Kim SS, Kim J, Kim S (2008) Effects of the operating parameters on the production of bio-oil in the fast pyrolysis of Japanese larch. Chem Eng J 143:124–132
Karmas E (1977) Preliminary studies on Arrhenius relationships of dehydration of proteins. In: Analytical Calorimetry. Springer, pp 81–90
Milosavljevic I, Oja V, Suuberg EM (1996) Thermal effects in cellulose pyrolysis: relationship to char formation processes. Ind Eng Chem Res 35:653–662
Moire L, Schmutz A, Buchala A, Yan B, Stark RE, Ryser U (1999) Glycerol is a suberin monomer. New experimental evidence for an old hypothesis. Plant Physiol 119:1137–1146
Bernards MA (2002) Demystifying suberin. Can J Bot 80:227–240
Pereira H (2015) The rationale behind cork properties: a review of structure and chemistry. BioResources 10:6207–6229
Beis S, Mukkamala S, Hill N et al (2010) Fast pyrolysis of lignins. BioResources 5:1408–1424
Marques AV, Pereira H (2013) Lignin monomeric composition of corks from the barks of Betula pendula, Quercus suber and Quercus cerris determined by Py–GC–MS/FID. J Anal Appl Pyrolysis 100:88–94
Budrugeac ÁP, Homentcovschi D, Segal E (2001) Critical considerations on the isoconversional methods. III. On the evaluation of the activation energy from non-isothermal data. J Therm Anal Calorim 66:557–565
Burnham AK, Dinh LN (2007) A comparison of isoconversional and model-fitting approaches to kinetic parameter estimation and application predictions. J Therm Anal Calorim 89:479–490
Criado J, Sánchez-Jiménez P, Pérez-Maqueda L (2008) Critical study of the isoconversional methods of kinetic analysis. J Therm Anal Calorim 92:199–203
Carrier M, Auret L, Bridgwater A, Knoetze JH (2016) Using apparent activation energy as a reactivity criterion for biomass pyrolysis. Energy Fuel 30:7834–7841
Wu W, Mei Y, Zhang L, Liu R, Cai J (2014) Effective activation energies of lignocellulosic biomass pyrolysis. Energy Fuel 28:3916–3923
Plis A, Kotyczka-Morańska M, Kopczyński M, Łabojko G (2016) Furniture wood waste as a potential renewable energy source. J Therm Anal Calorim 125:1357–1371
Acknowledgments
Forest Research Centre (CEF) is a research unit funded by the Fundação para a Ciência e a Tecnologia (FCT) (UIDB/00239/2020). A. Umut Sen acknowledges the postdoctoral fellowship from FCT (SFRH/BPD/87632/2012) and thanks the Chemical Engineering Department of Instituto Superior Técnico for the laboratorial use. The authors thank Joaquina Martins for her help in chemical analysis. F. G. Fonseca is a member of the BBW ForWerts Graduate Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Şen, A.U., Fonseca, F.G., Funke, A. et al. Pyrolysis kinetics and estimation of chemical composition of Quercus cerris cork. Biomass Conv. Bioref. 12, 4835–4845 (2022). https://doi.org/10.1007/s13399-020-00964-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00964-y