Abstract
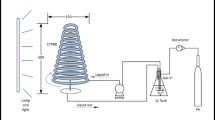
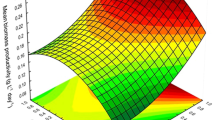
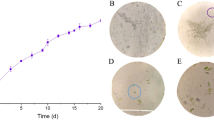
The impact and interaction of light irradiance strength (light intensities), lighting cycle (photoperiod), and aeration rate on biomass concentration and lutein production efficacy of the microalga Chlorella salina in a closed laboratory-scale airlift photobioreactor were investigated via the response surface method. Among the factors assessed, light intensity and aeration rate had significant influence on cell concentration, though a concurrent increment in light intensity noticeably decreased the lutein content. All the parameters were observed to be statistically significant. Best operating conditions for the growth of alga was evaluated to be as follows: light intensity, 200 μmol m−2 s−1; photoperiod, 12:12 h L D; and the aeration rate, 3 lpm. These conditions could substantially enhance the microalgal growth rate (0.82 day−1) and biomass production (665.89 mg). Specific lutein productivity and a recovery of 9.73 mg/L/day were achieved at a day light cycle of 16 h. According to the results of the experimental design, the optimum conditions led to a twofold increase in biomass and lutein productivity compared with unoptimized condition.




Similar content being viewed by others
References
Ceron MC, Campos I, Sanchez JF, Acien FG, Molina E, Fernandez-Sevilla JM (2008) Recovery of lutein from microalgae biomass: development of a process for Scenedesmus almeriensis biomass. J Agric Food Chem 56:11761–11766
Gong M, Bassi A (2016) Carotenoids from microalgae: a review of recent developments. Biotechnol Adv 34(8):1396–1412
Murray PM, Moane S, Collins C, Beletskaya T, Thomas OP, Duarte AWF, Nobre FS, Owoyemi IO, Pagnocca FC, Settle LD, McHugh E, Causse E, Perez-Lopez P, Feijoo G, Moreira MT, Rubiolo J, Leiros M, Botana LM, Walsh DJ (2013) Sustainable production of biologically active molecules of marine based origin. New Biotechnol 30:839–850
Walker TL, Purton S, Becker DK, Collet C (2005) Microalgae as bioreactors. Plant Cell Rep 24:629–641
Lamers PP, Van de Laak CCW, Kaasenbrood PS, Lorier J, Janssen M, De Vos RCH, Bino RJ, Wijffels RH (2010) Carotenoid and fatty acid metabolism in light-stressed Dunaliella salina. Biotechnol Bioeng 106:638–648
Lamers PP, Janssen M, De Vos RCH, Bino RJ, Wijffels RH (2012) Carotenoid and fatty acid metabolism in nitrogen-starved Dunaliellaalina, a unicellular green microalga. J Biotechnol 162:21–27
Wang B, Zarka A, Trebst A, Boussiba S (2003) Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process under high irradiance. J Phycol 39:1116–1124
Telfer A, Pascal A, Gall A (2008) Carotenoids in photosynthesis. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids. Carotenoids, vol 4. Birkhäuser Basel. pp. 265-308
Mulders KJM, Lamers PP, Martens DE, Wijffels RH (2014) Phototrophic pigment production with microalgae: biological constraints and opportunities. J Phycol 50:229–242
Carvalho AP, Silva SO, Baptista JM, Malcata FX (2011) Light requirements in microalgal photobioreactors: an overview of biophotonic aspects. Appl Microbiol Biotechnol 89:1275–1288
Brindley Alias C, Garcia-MaleaLopez MC, AcienFernandez FG, Fernandez Sevilla JM, Garcia Sanchez JL, Molina Grima E (2004) Influence of power supply in the feasibility of Phaeodactylum tricornutum cultures. Biotechnol Bioeng 87:723–733
Bitog JP, Lee IB, Lee CG, Kim KS, Hwang HS, Hong SW, Seo IH, Kwon KS, Mostafa E (2011) Application of computational fluid dynamics for modeling and designing photobioreactors for microalgae production: a review. Comput Electron Agric 76:131–147
Dineshkumar R, Dhanarajan G, Dash SK, Sen R (2015a) An advanced hybrid medium optimization strategy for the enhanced productivity of lutein in Chlorella minutissima. Algal Res 7:24–32
Dineshkumar R, Dash SK, Sen R (2015b) Process integration for microalgal lutein and biodiesel production with concomitant flue gas CO2 sequestration: a biorefinery model for healthcare, energy and environment. RSC Adv 5:73381–73394
Xie Y, Ho SH, Chen CN, Chen CY, Ng IS, Jing KJ, Chang JS, Lu Y (2013) Phototrophic cultivation of a thermo-tolerant Desmodesmus sp. for lutein production: effects of nitrate concentration, light intensity and fed-batch operation. Bioresour Technol 144:435–444
Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak MN (2008) Effects of light and nitrogen starvation on the content and composition of carotenoids of the green microalga Parietochloris incisa. Russ J Plant Physiol 55:455–462
Vaquero I, Mogedas B, Ruiz-Dominguez MC, Vega JM, Vílchez C (2014) Light- mediated lutein enrichment of an acid environment microalga. Algal Res 6:70–77
Walne PR (1970) Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria, and Mytilus. Fish Invest London Ser 2 26(5):1–62
Chang HL, Tseng YL, Ho KL, Shie SC, Wu PS, Hsu YT, Lee TM (2014) Reactive oxygen species modulate the differential expression of methionine sulfoxide reductase genes in Chlamydomonas reinhardtii by high light illumination. Plant Physiol 144:225–237
Chang HL, Hsu YT, Kang CY, Lee TM (2013) Nitric oxide down-regulation of carotenoid synthesis and PSII activity in relation to very high light-induced singlet oxygen production and oxidative stress in Chlamydomonas reinhardtii. Plant Cell Physiol 53:445–456
Polle JE, Niyogi KK, Melis A (2001) Absence of lutein, violaxanthin and neoxanthin affects the functional chlorophyll antenna size of photosystem-II but not that of photosystem-I in the green alga Chlamydomonas reinhardtii. Plant Cell Physiol 42:482–491
Tang H, Abunasser N, Garcia MED, Chen M, Simon Ng KY, Salley SO (2010) Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Appl Energy 88:3324–3330
Martinez ME, Jimnez JM, Yousfi FE (1999) Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour Technol 67:233–240
Barbosa MJ, Zijffers JW, Nisworo A, Vaes W, van Schoonhoven J, Wijffels RH (2005) Optimization of biomass, vitamins, and carotenoid yield on light energy in a flatpanel reactor using the A-stat technique. Biotechnol Bioeng 89(2):233–242
Ramos Tercero EA, Sforza E, Morandini M, Bertucco A (2014) Cultivation of Chlorella protothecoides with urban wastewater in continuous photobioreactor: biomass productivity and nutrient removal. Appl Biochem Biotechnol 172:1470–1485
Zijffers JWF, Schippers KJ, Zheng K, Janssen M, Tramper J, Wijffels RH (2010) Maximum photosynthetic yield of green microalgae in photobioreactors. Mar Biotechnol 12(6):708–718
Lv JM, Cheng LH, Xu XH, Zhang L, Chen HL (2010) Enhanced lipid production of Chlorella vulgaris by adjustment of cultivation conditions. Bioresour Technol 101:6797–6804
Wong YK, Yung KKL, Tsang YF, Xia Y, Wang L, Ho KC (2015b) Scenedesmus quadricauda for nutrient removal and lipid production in wastewater. Water Environ Res 87(12):2037–2044
Cordero BF, Obraztsova I, Couso I, Leon R, Vargas MA, Rodriguez H (2011) Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar Drugs 9(9):1607–1624
Guedes AC, Amaro HM, Malcata FX (2011) Microalgae as sources of carotenoids. Mar Drugs 9:625–644
Mahale VE, Chaugule BB (2013) Optimization of freshwater green alga Scenedesmus incrassatulus for biomass production and augmentation of fatty acids under abiotic stress conditions. Phykos 43(1):22–31
Sharma R, Singh GP, Sharma VK (2012) Effect of culture conditions on growth and biochemical profile of Chlorella vulgaris. J Plant Pathol Microbiol 3(5)
Anjos M, Fernandes BD, Vicente AA, Teixeira JA, Dragone G (2013) Optimization of CO2 bio-mitigation by Chlorella vulgaris. Bioresour Technol 139:149–154
Fan LH, Zhang YT, Cheng LH, Zhang L, Tang DS, Chen HL (2007) Optimization of carbon dioxide fixation by Chlorella vulgaris cultivated in a membrane-photobioreactor. Chem Eng Technol 30:1094–1099
Gai C, Zhang Y, Chen WT, Zhang P, Dong Y (2014) Energy and nutrient recovery efficiencies in biocrude oil produced via hydrothermal liquefaction of Chlorella pyrenoidosa. RSC Adv 4:16958
Del Campo JA, Moreno J, Rodriguez H, Vargas MA, Rivas J, Guerrero MG (2000) Carotenoid content of chlorophycean microalgae. Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J Biotechnol 76:51–59
Del Campo JA, Rodríguez H, Moreno J, Vargas MA, Rivas J, Guerrero MG (2001) Lutein production by Muriellopsis sp. in an outdoor tubular photobioreactor. J Biotechnol 81:289–295
Wei D, Chen F, Chen G, Zhang X, Liu L, Zhang H (2008) Enhanced production of lutein in heterotrophic Chlorella protothecoides by oxidative stress. Sci China C Life Sci 51:1088–1093
Sanchez JF, Fernandez-Sevilla JM, Acien FG, Ceron MC, Perez-Parra J, Molina-Grima E (2008) Biomass and lutein productivity of Scenedesmus almeriensis: influence of irradiance, dilution rate and temperature. Appl Microbiol Biotechnol 79:719–729
Sanchez JF, Fernandez-Sevilla JM, Acien FG, Rueda A, Perez-Parra J, Molina E (2008) Influence of culture conditions on the productivity and lutein content of the new strain Scenedesmus almeriensis. Process Biochem 43:398–405
Garbayo I, Cuaresma M, Vílchez C, Vega JM (2008) Effect of abiotic stress on the production of lutein and β-carotene by Chlamydomonas acidophila. Process Biochem 43:1158–1161
Shi XM, Zhang XW, Chen F (2000) Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzym Microb Technol 27(3–5):312–318
Ferreira SC, Bruns R, Ferreira H, Matos G, David J, Brandao G, da Silva E, Portugal L, Dos Reis P, Souza A (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597(2):179–186
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gayathri, S., Rajasree, S.R.R., Suman, T.Y. et al. Induction of β, ε-carotene-3, 3′-diol (lutein) production in green algae Chlorella salina with airlift photobioreactor: interaction of different aeration and light-related strategies. Biomass Conv. Bioref. 11, 2003–2012 (2021). https://doi.org/10.1007/s13399-019-00580-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00580-5