Abstract
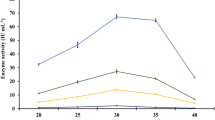
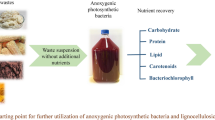
The present study documented the potential of two lignocellulosic wastewaters viz. corncob waste liquor (CWL) and paper mill effluent (PME) and a cellulosic waste (deoiled algae extract (DAE)) as feedstock for microbial oil production by Aspergillus awamori (MTCC 11639). Using cellulosic waste as substrate/feedstock for single cell oil (SCO) production reduced the overall cost of production and also accounts for simultaneous waste remediation. DAE showed improvement in biomass production with time, in terms of dry weight (12.8 g/l), followed by CWL (10.2 g/l) and PME (9.83 g/l). Total/neutral (T/N) lipid productivity recorded the same trend where higher T/N was obtained with DAE (35.4 and 18 %), followed by CWL (33.3 and 16.3 %) and PME (32.2 and 15 %). During the growth of fungi, efficient colour (78 %) along with good carbohydrate/chemical oxygen demand (COD) removal was registered, depicting the potential of A. awamori in utilizing the available carbon in waste. The presence of extracellular laccase enzyme in the fermentation medium is also an indicator that validates the observed colour removal and lignin degradation. Higher number of saturated fatty acid (SFA) than unsaturated fatty acid (USFA) in the fatty acid methyl esters (FAME) analysis indicates that the fungal oil obtained has properties similar to those of biodiesel. This study establishes a basis for fungal-based oil production utilizing cellulosic waste as primary feedstock. Integrating waste remediation with microbial oil production also forms an essential element in the present work, especially in the context of biorefinery.







Similar content being viewed by others
References
Sergeeva YE, Galanina LA, Andrianova DA, Feofilova EP (2008) Lipids of filamentous fungi as a material for producing biodiesel fuel. Appl Biochem Microbiol 44:523–527
Zheng Y, Yu X, Zeng J, Chen S (2012) Feasibility of filamentous fungi for biofuel production using hydrolysate from dilute sulfuric acid pretreatment of wheat straw. Biotechnol Biofuels 50:1–10
Venkata MS, Reddy MV, Chandra R, Venkata Subhash G, Devi MP, Srikanth S (2014) Bacteria for bioenergy: a sustainable approach towards renewability, biomass for sustainable applications. In: Gaspard S, Ncibi MC (ed) Pollution remediation and energy. RSC Publishers, Cambridge, p 251–289
Kyung OY, Ju J, Ahmad BR, Se HC, Seung WK, Chulhwan P (2013) Development of a Saccharomyces cerevisiae strain for increasing the accumulation of triacylglycerol as a microbial oil feedstock for biodiesel production using glycerol as a substrate. Biotechnol Bioeng 110:343–347
Venkata Subhash G, Venkata Mohan S (2011) Biodiesel production from isolated oleaginous fungi Aspergillus sp. using corncob waste liquor as a substrate. Bioresour Technol 102:9286–9290
Venkata Subhash G, Venkata Mohan S (2014) Lipid accumulation for biodiesel production by oleaginous fungus. Fuel 116:509–515
Ranjan KB (2014) Endophytic fungi: prospects in biofuel production. Proc Natl Acad Sci India Sect B Biol Sci. doi:10.1007/s40011-013-0294-3
Yang Y, Yan M, Hu B (2014) Endophytic fungal strains of soybean for lipid production. Bioenerg Res 7:353–361
Economou CN, Aggelis G, Pavlou S, Vayenas DV (2011) Modelling of single-cell oil production under nitrogen limited and substrate inhibition conditions. Biotechnol Bioeng 108:1049–1055
Papanikolaou S, Dimou A, Fakas S, Diamantopoulou P, Philippoussis A, Galiotou-Panayotou M (2011) Biotechnological conversion of waste cooking olive oil into lipid-rich biomass using Aspergillus and Penicillium strains. J Appl Mirobiol 110:1138–1150
Yousuf A, Sannino F, Addorisio V, Pirozzi D (2010) Microbial conversion of olive oil mill wastewaters into lipids suitable for biodiesel production. J Agric Food Chem 58:8630–8635
Chen XF, Huang C, Xiong L, Chen X, Chen Y, Maa LL (2012) Oil production on wastewaters after butanol fermentation by oleaginous yeast Trichosporon coremiiforme. Bioresour Technol 118:594–597
Pant D, Adholeya A (2010) Development of a novel fungal consortium for the treatment of molasses distillery wastewater. Environmentalist 30:178–182
Economou CN, Aggelis G, Pavlou S, Vayenas DV (2011) Single cell oil production from rice hulls hydrolysate. Bioresour Technol 102:9737–9742
Peng W, Huang C, Chen X, Xiong L, Chen X, Chen YM (2013) Microbial conversion of wastewater from butanol fermentation to microbial oil by oleaginous yeast Trichosporon dermatis. Ren Energy 55:31–34
Easterling ER, French WT, Hernandez R, Licha M (2009) The effect of glycerol as a sole and secondary substrate on the growth and fatty acid composition of Rhodotorula glutinis. Bioresour Technol 100:356–361
Subramaniam R, Dufreche S, Zappi M, Bajpai R (2010) Microbial lipids from renewable resources: production and characterization. J Ind Microb Biotechnol 37:1271–1287
Chtzifragkou S, Fakas M, Galiotou-Panayotou M, Komaitis G, Aggelis, Papanikolaou S (2010) Commercial sugars as substrates for lipid accumulation by Cunninghamella echinulata and Mortierella isabellina fungi. Eur J Lipid Sci Technol 112:1048–1057
Bellou S, Moustogianni A, Makri A, Aggelis S (2012) Lipids containing polyunsaturated fatty acids synthesized by zygomycetes grown on glycerol. Appl Biochem Biotechnol 166:146–158
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86:807–815, ISSN 0300-9084
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part II: technology and potential applications. Rev Eur J Lipid Sci Technol 113:1052–1073
Huang C, Chen XF, Xiong L, Chen XD, Ma LL, Chen Y (2013) Single cell oil production from low-cost substrates: the possibility and potential of its industrialization. Biotechnol Adv 129–139
Economou CN, Makri A, Aggelis G, Pavlou S, Vayenas DV (2010) Semi-solid state fermentation of sweet sorghum for the biotechnological production of single cell oil. Bioresour Technol 101:1385–1388
Huang C, Zong MH, Wu H, Liu QP (2009) Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresour Technol 100:4535–4538
Zhao X, Kong X, Hua Y, Feng B, Zhao ZB (2008) Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi. Eur J Lipid Sci Technol 110:405–412
Venkata Mohan S, Prathima Devi M, Mohanakrishna G, Amarnath N, Lenin Babu M, Sarma PN (2011) Potential of mixed microalgae to harness biodiesel from ecological water-bodies with simultaneous treatment. Bioresour Technol 102:1109–1117
Prathima Devi M, Venkata Subhash G, Venkata Mohan S (2012) Heterotrophic cultivation of mixed microalgae for lipid accumulation and wastewater treatment during sequential growth and starvation phases: effect of nutrient supplementation. Ren Energy 43:276–283
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association/American water works Association/Water Environment Federation, Washington, (DC, USA)
American Society for Testing and Materials (2002) Standard specification for biodiesel fuel (b100) blend stock for distillate fuels. Designation D6751–02. ASTM International, West Conshohocken
Morris DL (1948) Quantitative determination of carbohydrates with Dreywood’s anthrone reagent. Science 107:254–255
Kimura K, Yamaoka M, Kamisaka Y (2004) Rapid estimation of lipids in oleaginous fungi and yeasts using Nile red fluorescence. J Microbiol Methods 56:331–338
Katre G, Joshi C, Khot M, Zinjarde S, RaviKumar A (2012) Evaluation of single cell oil (SCO) from a tropical marine yeast Yarrowia lipolytica NCIM 3589 as a potential feedstock for biodiesel. AMB Expr 36:1–14
Khot M, Kamat S, Zinjarde S, Pant A, Chopade B, RaviKumar A (2012) Single cell oil of oleaginous fungi from the tropical mangrove wetlands as a potential feedstock for biodiesel. Microb Cell Factories 11:71
Leonowicz A, Grzywnowicz K (1983) Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as a substrate. Enzym Microb Technol 3:55–58
Leonardo S, Silvia G, Giovanni S, Pier GP (1999) Laccase catalyzed-oxidative coupling of 3-methyl 2-benzothiazolinone hydrazone and methoxyphenols. Enzym Microb Technol 25:285–289
Krishna Prasad K, Venkata Mohan S, Vijaya Bhaskar Y, Ramanaiah SV, Lalit Babu V, Pati BR, Sarma PN (2005) Laccase production using Pleurotus ostreatus 1804 immobilized on PUF cubes in batch and packed bed reactors: influence of culture conditions. J Microbiol 301–307
Nguyen, Minh T, Seung PC, Jinwon L, Jae HL, Sang JS (2009) Hydrothermal acid pretreatment of chlamydomonas reinhardtii biomass for ethanol production. J Microbiol Biotechnol 19:161–166
Granger LM, Perlot P, Goma G, Pareilleux A (1993) Efficiency of fatty acid synthesis by oleaginous yeasts: prediction of yield and fatty acid cell content from consumed C/N ratio by a simple method. J Biochem Microbiol Technol Eng 42:1151–1156
Chatzifragkou A, Makri A, Belka S, Bellou M, Mavrou M, Mastoridou P (2011) Papanikolaou Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 36:1097–1108
Li Y, Zhao Z, Bai F (2007) High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-bach culture. Enzym Microbiol Technol 41:312–317
Park WS, Murphy PA, Glatz BA (1990) Lipid metabolism and cell composition of the oleaginous yeast Apiotrichum curvatum grown at different carbon to nitrogen ratios. Can J Microbiol 36:318–326
Wu S, Hu C, Jin G, Zhao X, Zhao ZK (2010) Phosphate-limitation mediated lipid production by Rhodosporidium toruloides. Bioresour Technol 101:6124–6129
Papanikolaou S, Aggelis G (2003) Modeling lipid accumulation and degradation in Yarrowia lipolytica cultivated on industrial fats. Curr Microbiol 46:398–402
Huang C, Chen XF, Xiong L, Chen XD, Maa LL (2012) Oil production by the yeast Trichosporon dermatis cultured in enzymatic hydrolysates of corncobs. Bioresour Technol 110:711–714
Fakas S, Panayotou MG, Papanikolaou S, Komaitis M, Aggelis G (2007) Compositional shifts in lipid fractions during lipid turnover in Cunninghamella echinulata. Enzym Microb Technol 40:1321–1327
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. Eur J Lipid Sci Technol 113:1031–1051
Ratledge C (2002) Regulation of lipid accumulation in oleaginous micro-organisms. Biochem Soc Trans 30:1047–1050
Somerville C, Browse J, Jaworski JG, Ohlrogge JB (2000) Lipids. In: Buchanan B, Gruissem W, Jones R (eds) Biochemistry and molecular biology of plants. American Society of Plant Physiology, Rockville, pp 465–527
Schwender J, Ohlrogge JB (2002) Probing in vivo metabolism by stable isotope labeling of storage lipids and proteins in developing Brassica napus embryos. Plant Physiol 130:347–361
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–44
Wältermann M, Hinz A, Robenek H, Troyer D, Reichelt R, Malkus U, Galla HJ, Kalscheuer R, Stöveken T, Landenberg P, Steinbüchel A (2005) Mechanism of lipid-body formation in prokaryotes: how bacteria fatten up. Mol Microbiol 55:750–763
Ali M, Sreekrishnan TR (2001) Aquatic toxicity from pulp and paper mill effluents: a review. Adv Environ Res 5:175–196
Lacorte S, Latorre A, Barceló D, Rigol A, Malmqvist A, Welander T (2003) Organic compound in paper-mill process waters and effluents. Trends Anal Chem 22:725–737
Wong SS, Teng TT, Ahmada AL, Zuhairi A, Najafpour G (2006) Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J Hazard Mater 135:378–388
Singh P, Thakur IS (2004) Removal of colour and detoxification of pulp and paper mill effluent by microorganisms in two step bioreactor. J Sci Ind Res 63:944–948
Homada MF, Haddad AI, Abd-El-Bary MF (1987) Treatment of phenolic wastes in an aerated submerged fixed-film (ASFF) bioreactor. J Biotechnol 5:279–292
Adinarayana K, Antonio B, Plou FJ, Alcalde M (2007) Fungal laccase – a versatile enzyme for biotechnological applications. Commun Curr Res Educ Top Trends Appl Microbiol 233–245
Rossi M, Amaretti A, Raimondi S, Leonardi A (2011) Getting lipids for biodiesel production from oleaginous fungi, biodiesel - feedstocks and processing technologies, Dr. Margarita Stoytcheva (Ed.), ISBN: 978-953-307-713-0
Leeuwen JV, Rasmussen ML, Sankaran S, Koza CR, Erickson DT, Mitra D, Jin B (2012) Fungal treatment of crop processing wastewaters with value-added co-products sustainable bioenergy and bioproducts. Green En and Technol 13–44
Jin B, Van Leeuwen J, Patel B, Doelle HW, Yu Q (1999) Production of fungal protein and glucoamylase by Rhizopus oligosporus from starch processing wastewater. Process Biochem 34:59–65
Guimarães C, Porto P, Oliveira R, Mota M (2005) Continuous decolourization of a sugar refinery wastewater in a modified rotating biological contactor with Phanerochaete chrysosporium immobilized on polyurethane foam disks. Process Biochem 40:535–540
Scandellari F, Hobbie EA, Ouimette AP, Stucker VK (2009) Tracing metabolic pathways of lipid biosynthesis in ectomycorrhizal fungi from position-specific 13C-labelling in glucose. Environ Microbiol 11:3087–3095
Fakas S, Papanikolaou S, Batsos A, Panatoyou MG, Mallouchos A, Aggelis G (2009) Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina. Biomass Bioenerg 33:573–580
Kang SW, Park YS, Lee JS, Hong SI, Kim SW (2004) Production of cellulases and hemicellulases by Aspergillus niger KK2 from lignocellulosic biomass. Bioresour Technol 91:153–156
Acknowledgments
The authors wish to thank Director, CSIR-IICT for his support and encouragement in carrying out this work. GVS acknowledges Council for Scientific and Industrial Research (CSIR), New Delhi for providing research fellowship. Research was supported by the CSIR by providing the funding in the form of XII five year network project on ‘(SETCA)-CSC-0113’ and SAHYOG-EU-FP7-KBBE project funded by DBT (BT/IN/EU/07/PMS/2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subhash, G.V., Mohan, S.V. Sustainable biodiesel production through bioconversion of lignocellulosic wastewater by oleaginous fungi. Biomass Conv. Bioref. 5, 215–226 (2015). https://doi.org/10.1007/s13399-014-0128-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-014-0128-4