Abstract
The synthesis of multifunctional composites still relies on the use of conventional methods. However, these methods are expensive, time consuming and require high volumes of reducing agents which are often toxic. In this study, composites of bentonite-supported silver nanoparticles were prepared comparatively by the conventional heating method and the rapid microwave method; and their antibacterial activity was investigated against Escherichia coli and Staphylococcus aureus. The crystalline nature of the composites was evaluated by X-ray diffraction (XRD), while transmission electron microscope (TEM) coupled with energy-dispersive spectroscope was used for morphology and elemental analysis, respectively. Surface area and pore size analysis of the composites were conducted by the Brunauer, Emmett and Teller analyzer. TEM images revealed successful synthesis of the composites with a better dispersion of the nanoparticles achieved through microwave, where nanoparticle sizes were 6–38 nm and 9–56 nm by the conventional method. It is worth noting that the composites were prepared in less than 30 min using microwave as compared to 2 h of the conventional method. The XRD spectra confirmed the formation of silver and not any other impurities of the metal. These results revealed that, although the two methods are comparable, microwave method is efficient and time saving and can, therefore, synthesize composites with well-dispersed and narrow distributed nanoparticles. The antibacterial results demonstrated that the prepared composites are effective in the inactivation of various bacteria. These composites could be applied in water treatment, wound dressing, packaging, etc.
Similar content being viewed by others
1 Introduction
Silver is a well-known metal with strong inhibitory and bactericidal effects on a broad spectrum of bacteria. Due to its properties and widespread applications, silver has received extensive attention with the aim of functionally improving its properties [1,2,3]. Furthermore, silver nanoparticles (AgNPs) are intensively being studied due to their high surface area, size-dependent properties and exceptional antibacterial properties than bulk silver [4,5,6,7,8]. However, because of their inherently small sizes, nanoparticles tend to aggregate when they are utilized alone which hinders some of their properties and their dangers toward the environment are not fully understood. The literature has shown that supports are feasible solution to prevent agglomeration between the nanoparticles and ensures a consistent distribution of the nanoparticles throughout the supporting material which is essential for composite performance. Composites of metals or metal oxides with various supporting materials are specifically attractive due to the increasing demand for high-performance materials.
Among the materials used to support nanoparticles for various applications are clays. Clays are environmentally friendly, have high chemical stability, available at low cost and in great quantity. Clays are particularly active materials because of their high surface area, which is advantageous for supporting nanoparticles. Additionally, because clays are non-toxic, clay supported with nanoparticles is promising to have widespread applications [9,10,11,12]. Bentonite is a smectite clay which is mainly composed of montmorillonite and other non-clay minerals. The clay consists of two tetrahedral sheets which are separated by an octahedral sheet [11, 13, 14]. Various synthesis protocols have been established for the structural modification and composite preparation of clays. The methods are predominantly broad since the use of non-toxic solvents, biodegradable materials and low-cost chemicals are central to the synthesis process. These methods include and are not limited to chemical reduction [15, 16], laser ablation [17, 18], microemulsion [19], UV irradiation [20], microwave [21, 22], etc. Although these methods are capable of synthesizing composites, they are expensive, time consuming, requires high volumes of reducing agents which are often toxic and controlling the nanoparticle distribution, size and morphology which is paramount to the performance of the composites is not always feasible.
As a rapid and simple method, microwave method is receiving attention for the synthesis of various composites. Compared to conventional methods, microwave has several advantages including reduced reaction time, enhanced reaction rate and homogenous nanoparticles with narrow size distribution of the nanoparticles [23]. Another advantage of microwave is that process optimization to improve the composite properties is easier which is not always feasible when using conventional methods. Parameters that have an influence on the morphology, size and particle distribution such as reaction time, power, temperature and magnetic stirring system are controllable when using microwave method. Composite reactions which would normally take hours with conventional methods could easily take minutes with microwave method. Therefore, the use of microwave method for nanoparticles and composites preparation is considered attractive because of the reduced time, lower temperatures, high efficiency and environmentally friendly.
In this study, the preparation of bentonite supported with AgNPs, their characterization and antibacterial activity is reported. The composites were prepared comparatively by using microwave method and conventional heating method. Thus, the objective of the study is to demonstrate a better synthesis route for clay composites by using a more efficient method with less toxic reducing solvents and reduced preparation time. Cutting-edge characterization equipment such as TEM, EDS, XRD, dynamic light scattering (DLS) particle size analyzer and BET surface area and pore measurement were used for the analysis of the composites. The antibacterial efficacy of the composites was evaluated with E. coli and S. aureus as model bacterial strains.
2 Experimental
2.1 Materials
Bentonite which was provided by Ecca Holdings from South Africa was used as a support for AgNPs. Sulfuric acid (H2SO4, 98%) and silver nitrate (AgNO3, 99.98%) were all analytical grade and were used as received from Sigma-Aldrich. Distilled water was employed to prepare all aqueous solutions. Escherichia coli (E. coli, ATCC 25922), a gram-negative bacteria, and Staphylococcus aureus (S. aureus, ATCC 25923), a gram-positive bacteria, were used as model bacterial strains and were obtained from the American Type Culture Collection.
2.2 Synthesis Methods
2.2.1 Conventional Heating Method
Twenty gram (20 g) of < 150 µm sieved clay was refluxed with 3 M H2SO4 solution at 90 °C for 2 h (2 h was found to be the optimum reaction time). After the reaction, the clay was washed several times to remove any excess impurities, filtered and dried overnight at 105 °C. The clay was then crushed to a size < 150 µm. Subsequently, 0.1 M AgNO3 solution was prepared, and 500 ml added to 15 g of the treated clay. The mixture was refluxed for 2 h at 80 °C. The resulting mixture was washed several times to remove any excess impurities, filtered and dried overnight at 60 °C. The dried composite was then crushed to a size < 150 µm.
2.2.2 Microwave Synthesis Method
Firstly, a sieve of < 150 µm aperture was used to sieve the clay. Part of the clay was refluxed with 3 M H2SO4 solution at 90 °C for 20 min using microwave reactor at 500 W. After the reaction, the clay was washed several times, filtered and dried overnight at 105 °C. The clay was then crushed to a size < 150 µm. Subsequently, 0.1 M AgNO3 solution was prepared, and 50 ml added to 1.5 g of the treated clay. The mixture was heated for 20 min using microwave at 80 °C and 500 W. The resulting mixture was washed several times, filtered and dried overnight at 60 °C. The dried composite was then crushed to a size < 150 µm.
2.3 Characterization
The elemental composition and surface morphology of pristine clay and the composites were investigated by transmission electron microscope (TEM) JEOL 2100 F that was run at 200 kV and also coupled with energy-dispersive spectroscope (EDS). The powdered samples were dispersed in an ethanol solution for few minutes, and a drop was placed on a holey carbon-supported copper grids. Powder X-ray diffraction (XRD, PAnalytical XPERT-PRO diffractometer) analyses using Ni filtered CuKα radiation (k = 1.5406 Å) with a variable slit at 40 mA/45 kV were used to evaluate the crystalline phases of the materials. The diffraction measurements were done at a scan range of 2θ = 0–90°. Simultaneous evaluation of the specific surface area and pores sizes of all the samples was conducted using Brunauer, Emmett and Teller (BET) analyzer provided by Micromeritics (TRISTAR 3000) using N2 adsorption method at low temperatures. Before the measurements, all samples were degassed under constant flow of N2 gas overnight at 50 °C to remove the adsorbed contaminants from the surfaces of the samples. Horiba Dynamic Light Scattering Particle Size Analyzer LB-550 was used to measure the particle size distribution. To evaluate the stability of the nanoparticles on the clays, composite samples were analyzed with inductively coupled plasma atomic emission spectroscopy (ICP-AES, PerkinElmer, USA).
2.4 Antibacterial Activity
E. coli and S. aureus were used as model strains to evaluate the antibacterial efficiency of the prepared composites. Nutrient agar and broth were used for the growth of the selected bacteria. Prior to the evaluation, sterile nutrient broth in 100 ml bottle was inoculated with a loop-full of each bacteria culture and incubated in a shaking incubator for 24 h at 37 °C and 120 rpm. To assess the bacterial growth, 100 µl of each bacteria suspension was inoculated into nutrient agar plates and incubated at 37 °C for 24 h. Thereafter, 100 ml of nutrient broth was inoculated with 3–5 colonies of each species and incubated again. A quantity of 100 µl of each bacterial species was inoculated into nutrient agar plates at room temperature and left to settle. Pellets of 9 mm were made from pristine clay, acid-treated clay and composites. The pellets were carefully put on the agar and incubated for 24 h. Finally, the inhibition zone was measured, and representative photographs were taken. The bacterial evaluation was done in triplicates, and averages are given.
2.5 Leaching Analysis
To test the stability of the nanoparticles on the clay, leaching tests were conducted on the composites. A 50 ml sampling bottle was filled with distilled water, and 500 mg of each composite was added. The composites were subjected to vigorous shaking using a bath shaker set at 30 °C and 200 rpm. Leachates were collected at different time intervals, filtered and analyzed with ICP-AES.
3 Results and Discussion
3.1 TEM, Particle Size and EDS Analysis
Figure 1 shows HRTEM and TEM representative micrographs of pristine clay, acid-activated clay and clay–AgNP composites, and the corresponding particle size distribution is given in Fig. 2. Figure 1a and b provides proof of pristine clay with its smooth and clear surfaces. Following acid treatment by microwave (MW) (Fig. 1c and d), the clay retains its smooth morphology while that treated by the conventional method (CM) (Fig. 1e and f) appears to be rough and with defects. It is important to note that although acid-treated clay by conventional method showed defects, clays treated by microwave showed no obvious evidence of damage or shortening of length following the treatment. Less defects on the clay surfaces can serve as potential active sites for supporting nanoparticles; however, severe defects can destroy the clay structure and hinder the possibility of supporting nanoparticles. Additionally, Fig. 1g–i and Fig. 1j–l is evident of the dispersed AgNPs on the clay surfaces by microwave and conventional method, respectively. Both methods demonstrated a comparative homogeneous distribution of the nanoparticles with no obvious aggregation observed. When nanoparticles are synthesized alone, they often aggregate and become less stable; hence, it is imperative to use stabilizing agents or supporting materials. Furthermore, the findings of the study demonstrated that clays are crucial supporting materials for nanoparticles and their particle sizes can be optimized. The measured sizes by TEM were between 6–38 nm for microwave and 9–56 nm for conventional method. The particle size measurements for microwave composites were in agreement with those measured with the particle size analyzer (Fig. 2a), whereas for the conventional method, a wide distribution was observed with a maximum particle size of around 130 nm (Fig. 2b). Agglomeration of nanoparticles was not obviously observed on TEM images, but the size of the nanoparticles suggested that agglomeration could have been possible since there were larger particles measured. Small and narrow distributed nanoparticle sizes are crucial characteristics for displaying enhanced properties, and microwave method has shown to be advantageous in this regard and is also promising for large-scale production. The EDS (Fig. 3) quantitative analysis confirmed the presence of AgNPs in the composites which contained about 19 and 6 wt% of Ag in the composites prepared by microwave and conventional method, respectively. These results reveal that a high percentage of AgNPs was obtained with samples prepared by microwave method. The results may also be explained and correlated to TEM morphology. TEM images by the conventional method showed some obvious roughness on the clay surfaces which might be due to the defects on the surfaces. The defects which mostly act as active sites for nanoparticles could be too damaged to provide a strong attachment of the nanoparticles, hence, the low AgNPs content as observed from EDS analysis.
3.2 Surface Area and Pore Size Analysis
BET specific surface area (SBET) and pore size measurements are given in Table 1. Pristine clay was observed to have an SBET of 56 m2g−1, which increased as the clay was acid-treated. Samples acid-treated using conventional method have shown a higher SBET of 231 m2g−1 while those treated using microwave method had an SBET of 150 m2g−1. A significant increase in the SBET from pristine clay to acid-treated samples is attributed to the impurities removed, and/or the replacement of interchangeable cations found between the tetrahedral and octahedral layers of the clay, hence, exposing the edges [24, 25]. The decreased SBET (106 m2g−1) of clay composites prepared by microwave is due to the relatively higher amount of AgNPs dispersed on the surfaces of the clay. However, it is worth mentioning that the SBET by microwave was larger than that of pristine clay, which is still high enough and could provide the composite with enhanced properties than the individual materials. Compared with the conventional composites, the microwave composites showed a decreased SBET while that of the former increased (239 m2g−1). A decrease is often expected when nanoparticles are deposited on support materials with high surface areas because they fill the surfaces. But for composites prepared by the conventional method, the SBET had a nominal increase. The increase might be due to the low attachment of AgNPs on the clay surfaces, where instead of filling the surfaces, the less deposited nanoparticles were exposed on the clay surface edges and, therefore, increased the SBET. For both composites, a decrease in pore sizes was observed which is a usual occurrence caused by the filling up of pores by the nanoparticles [24].
3.3 XRD Analysis
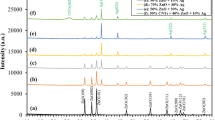
Powder XRD studies were carried out (Fig. 4) to establish the formation of AgNPs and the crystallinity of the composites. The presence of clays was indicated by peaks appearing at
2θ = 6, 20 and 26° [13, 26,27,28]. Other peaks assigned to the clay were observed at 35, 50 and 62°, which were observed in all the samples. The peak at 2θ = 6° for the acid-treated clay was observed with an increased and sharper intensity, while the other peaks remained the same as compared to pristine clay. However, this peak (2θ = 6°) was observed with less intensity for both clay–AgNP composites. The presence of Ag on the clay was correlated to a typical diffraction peak at 39° which showed that AgNPs were successfully formed on the clay surfaces. This peak was observed with a very low intensity in both the composites prepared by microwave and conventional method, unlike what was observed from EDS analyses. The low intensity of the AgNPs diffraction peaks in the clay was also observed by Magana et al. [28] and Shameli et al. [29], where they attributed the peak to the incorporation of AgNPs into the framework of the montmorillonite [30] and the even distribution of AgNPs in the interlayer and surface of montmorillonite. According to a reported study by Shameli et al. [31], the diffraction peaks of silver could appear at 2θ values of 38, 44 and 65°. The 2θ = 39° peak which was observed in the current study is associated with the simple face-centered cubic (fcc) silver crystallographic planes (111) [28, 29].
3.4 Bacterial Activity
The bacterial activity of pristine clay and composites was evaluated using two bacterial strains. Figure 5 gives representative photographs taken after the antibacterial evaluation through the disk diffusion method, and Table 2 provides a summary of the findings. The clay used in the study in its pristine form did not show any antibacterial activity as observed from Fig. 5; with the pellet at the center denoted “1.” The antibacterial activity of natural clays is variable, since no naturally occurring clay minerals are exactly the same due to the variations in their mineralogical and geological compositions. However, the acid-treated clays by both methods (microwave denoted “2” and conventional “3”) showed an inhibition zone around the pellet. This is due to the active sites introduced on the clay during the acid-activation, and as a result, the acidic environment contributes to the response and availability of toxic metal ions of the clays; hence, an antibacterial activity is observed. The composite results by both methods (microwave denoted “4 and 5” and conventional denoted “6 and 7’) exhibited excellent antibacterial activity toward both E. coli and S. aureus. It was observed that composites prepared by microwave had a higher inhibition zone compared to those prepared by the conventional method. This could be correlated to the small particles sizes and well-dispersed AgNPs. It is said that AgNPs with small sizes have superior antibacterial properties as compared to those with larger sizes [1,2,3]. Another notable observation is the antibacterial efficacy of E. coli and S. aureus, which could be justified by their structural characteristics. Gram-positive bacteria have a thick cell wall which makes the penetration of the silver ions (Ag+) difficult, and at a low rate, whereas gram-negative bacteria are characterized by a thin cell wall which makes the bacteria more vulnerable to foreign objects. The antibacterial effect of AgNPs is attributed to the Ag+ ions released, in which the release is associated to the surface energy of the nanoparticles [1,2,3]. Other possible mechanisms of AgNPs inactivation may include the generation of reactive oxygen species, attachment of AgNPs on the cell wall of the bacteria and an increased permeation rate within the bacterial cell [1,2,3, 32, 33]. A study by Krishnan and Mahalingam [32] stated that bacteria cell walls are negatively charged as the cell surfaces are composed of functional groups such as phosphate, hydroxyl and carboxyl in their lipid bilayer. Hence, cations such as Ag+ from composite materials can easily attach to the cell surfaces and disrupt the cell metabolism, causing cell death. Similar results were observed by Moosa et al. [33], where they prepared nanocomposites of AgNPs/kaolin and evaluated the bacterial activity with E. coli and Enterococcus faecalis (E. faecalis). Their results showed that the interaction between the AgNPs and the negatively charged cell wall caused a disruption of the cell membrane leading to cell leakage and consequently cell death. Therefore, the results of the current study also indicate that the composites are promising antibacterial material applicable in water treatment, pharmaceutical, biomedical or as reinforcement materials for packaging materials.
3.5 Leaching Analysis
The stability of the nanoparticles on the clays was analyzed with ICP, and the results are given in Table 3. The results show that the amount of AgNPs that leached into the water increased with more shaking and contact time. However, the leached nanoparticles were still within the acceptable limits as recommended by the World Health Organization (WHO) standards [34]. The acceptable limits for Ag as given by the WHO are set to be below 0.005 mg/l. Additionally, the WHO has suggested that drinking water treated with Ag for disinfection may have the concentration of Ag above 50 µg/l. This is because daily estimates for Ag consumption per person have been set at 7 µg. Therefore, these results indicate that the composites are safe to use for consumer products including water treatment, and the stability of AgNPs is positive.
4 Conclusions
In this study, clay-supported AgNPs were prepared comparatively by microwave and conventional method, and their antibacterial activity was evaluated. Uniform dispersion on the clay surfaces was observed with small nanoparticle sizes (6–38 nm by microwave and 9–56 nm by conventional method). Microwave method was observed to significantly reduce the required reaction time of the composites. Composites were synthesized in less than 30 min as compared to 2 h of the conventional method. The antibacterial studies indicated that both composites are suitable to inactivate the bacteria tested. Furthermore, both composites showed a more favorable inhibition zone on E. coli than on S. aureus. This may be due to the thin cell wall of the gram-negative bacteria making it more vulnerable as compared to the gram-positive bacteria which has a thick cell wall making it more resistant to antibacterial agents. Additionally, the antibacterial properties of the composites are promising for applications in water treatment, pharmaceutical, biomedical or as reinforcement materials for packaging materials.
References
Yuan, Q.; Golden, T.A.: A novel method for synthesis of clay/polymer stabilized silver nanoparticles. Surf. Interfaces 20, 100620 (2020). https://doi.org/10.1016/j.surfin.2020.100620
Noori, A.; Neree, A.T.; Megoura, M.; Mateescu, M.A.; Azzouz, A.: Insights into the metal retention role in the antibacterial behavior of montmorillonite and cellulose tissue-supported copper and silver nanoparticles. RSC Adv. 11, 24156–24171 (2021). https://doi.org/10.1039/D1RA02854E
Sharma, N.T.; Vishwakarma, J.; Rai, S.; Alomar, T.S.; Almasoud, A.; Bhattarai, A.: Green route synthesis and characterization techniques of silver nanoparticles and their biological adeptness. ACS Omega 7, 27004–27020 (2022). https://doi.org/10.1021/acsomega.2c01400
Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A.: Synthesis, characterization and biomedical application of silver nanoparticles. Materials 15, 427 (2022). https://doi.org/10.3390/ma15020427
Chang, C.H.; Lee, Y.H.; Liao, Z.H.; Chen, M.H.C.; Peng, F.C.; Lin, J.J.: Composition of nanoclay supported silver nanoparticles in furtherance of mitigating cytotoxicity and genotoxicity. PLoS ONE 16, e0247531 (2021). https://doi.org/10.1371/journal.pone.0247531
Chang, C.H.; Tsai, L.H.; Lee, Y.C.; Yao, W.C.; Lin, J.J.: Synergistic effects of silicate-platelet supporting Ag and ZnO, offering high antibacterial activity and low cytotoxicity. Int. J. Mol. Sci. 24, 7024 (2023). https://doi.org/10.3390/ijms24087024
Sana, S.S.; Haldhar, R.; Parameswaranpillai, J.; Chavali, M.; Kim, S.C.: Silver nanoparticles-based composite for dye removal: a comprehensive review. Clean. Mater. 6, 100161 (2022). https://doi.org/10.1016/j.clema.2022.100161
Asmare, Z.G.; Aragaw, B.A.; Atlabachew, M.; Wubieneh, T.A.: Kaolin-supported silver nanoparticles as an effective catalyst for the removal of methylene blue dye from aqueous solutions. ACS Omega 8, 480–491 (2023). https://doi.org/10.1021/acsomega.2c05265
Zhang, H.; Xia, M.; Wang, F.; Li, P.; Shi, M.: Adsorption properties and mechanism of montmorillonite modified by two Gemini surfactants with different chain lengths for three benzotriazole emerging contaminants: experimental and theoretical study. Appl. Clay Sci. 207, 106086 (2021). https://doi.org/10.1016/j.clay.2021.106086
Amari, A.; Mohammed, A.F.; Mohammedsaleh, K.K.; Salem, A.N.; Tahoon, M.A.; Ben, R.F.: Clay-polymer nanocomposites: preparations and utilization for pollutants removal. Materials 14, 1365 (2021). https://doi.org/10.3390/ma14061365
Nomicisio, C.; Ruggeri, M.; Bianchi, E.; Vigani, B.; Valentino, C.; Aguzzi, C.; Viseras, C.; Rossi, S.; Sandri, G.: Natural and synthetic clay minerals in the pharmaceutical and biomedical fields. Pharmaceutics 15, 1368 (2023). https://doi.org/10.3390/pharmaceutics15051368
de Lucas-Gil, E.; Menéndez, J.; Pascual, L.; Fernández, J.F.; Rubio-Marcos, F.: The benefits of the ZnO/clay composite formation as a promising antifungal coating for paint applications. Appl. Sci. 10, 1322 (2020). https://doi.org/10.3390/app10041322
Korichi, S.; Elias, A.; Mefti, A.: Characterization of smectite after acid activation with microwave irradiation. Appl. Clay Sci. 42, 432–438 (2009). https://doi.org/10.1016/j.clay.2008.04.014
Amadio, T.D.M.; Hotza, D.; Neto, J.B.R.; Blosi, M.; Costa, A.L.; Dondi, M.: Bentonites functionalized by impregnation with TiO2, Ag, Pd and Au nanoparticles. Appl. Clay Sci. 146, 1–6 (2017). https://doi.org/10.1016/j.clay.2017.05.028
Asamoah, R.B.; Yaya, A.; Nbelayim, P.; Annan, E.; Onwona-Agyeman, B.: Development and characterization of clay-nanocomposites for water purification. Materials (Basel) 13, 3793 (2020). https://doi.org/10.3390/ma13173793
Zhang, H.; Hodges, C.S.; Mishra, P.K.; Yoon, J.Y.; Hunter, T.N.; Lee, J.W.; Harbottle, D.: Bio-inspired preparation of clay–hexacyanoferrate composite hydrogels as super adsorbents for Cs+. ACS Appl. Mater. Interfaces 12, 33173–33185 (2020). https://doi.org/10.1021/acsami.0c06598
Jaleh, B.; Mousavi, S.S.; Sajjadi, M.; Eslamipanah, M.; Maryaki, M.J.; Orooji, Y.; Varma, R.S.: Synthesis of bentonite/Ag nanocomposite by laser ablation in air and its application in remediation. Chemosphere 315, 137668 (2023). https://doi.org/10.1016/j.chemosphere.2022.137668
Hossain, S.I.; Bajrami, D.; Sportelli, M.C.; Picca, R.A.; Volpe, A.; Gaudiuso, C.; Ancona, A.; Gentile, L.; Palazzo, G.; Ditaranto, N.; Mizaikoff, B.; Cioffi, N.: Preparation of laser-ablated Ag nanoparticle–MMT clay-based beeswax antibiofilm coating. Antibiotics. 12, 194 (2023). https://doi.org/10.3390/antibiotics12020194
Bahranowski, K.; Gawel, A.; Klimek, A.; Michalik-Zym, A.; Napruszewska, B.D.; Nattich-Rak, M.; Rogowska, M.; Serwicka, E.M.: Influence of purification method of Na-montmorillonite on textural properties of clay mineral composites with TiO2 nanoparticles. Appl. Clay Sci. 140, 75–80 (2017). https://doi.org/10.1016/j.clay.2017.01.032
Zang, L.; Qiu, J.; Yang, C.; Sakai, E.: Preparation and application of conducting polymer/Ag/clay composite nanoparticles formed by in situ UV-induced dispersion polymerization. Sci. Rep. 6, 20470 (2016). https://doi.org/10.1038/srep20470
Motshekga, S.C.; Ray, S.S.; Onyango, M.S.; Momba, M.N.B.: Microwave-assisted synthesis, characterization and antibacterial activity of Ag/ZnO nanoparticles supported bentonite clay. J. Hazard. Mater. 262, 439–446 (2013). https://doi.org/10.1016/j.jhazmat.2013.08.074
Peng, H.; Yang, A.; Xiong, J.: Green, microwave-assisted synthesis of silver nanoparticles using bamboo hemicelluloses and glucose in an aqueous medium. Carbohydr. Polym. 91, 348–355 (2013). https://doi.org/10.1016/j.carbpol.2012.08.073
Hao, C.; Du, Y.; Li, L.: Microwave-assisted heating method for the decoration of carbon nanotubes with zinc sulphide nanoparticles. J. Dispers. Sci. Technol. 30, 691–693 (2009). https://doi.org/10.1080/01932690701688821
Zhang, J.; Wang, Q.; Chen, H.; Wang, A.: XRF and nitrogen adsorption studies of acid-activated palygorskite. Clay Miner. 45, 145–156 (2010). https://doi.org/10.1180/claymin.2010.045.2.145
Motlagh, M.M.K.; Youzbashi, A.A.; Rigi, Z.A.: Effect of acid activation on structural and bleaching properties of a bentonite. Iran. J. Mater. Sci. Eng. 8, 50–56 (2011)
Ajemba, R.O.: Alteration of bentonite from Ughelli by nitric acid activation: kinetics and physicochemical properties. Indian J. Sci. Technol. 6, 4074–4083 (2013). https://doi.org/10.17485/ijst/2013/v6i2.20
Bekele, W.; Faye, G.; Fernandez, N.: Removal of nitrate ion from aqueous solution by modified Ethiopian bentonite clay. Int. J. Res. Pharm. Chem. 4, 192–201 (2014)
Magana, S.M.; Quintana, P.; Aguilar, D.H.; Toledo, J.A.; Ángeles-Chávez, C.; Cortés, M.A.; León, L.; Freile-Pelegrín, Y.; López, T.; Torres Sánchez, R.M.: Antibacterial activity of montmorillonites modified with silver. J. Mol. Catal. A Chem. 281, 192–199 (2006). https://doi.org/10.1016/j.molcata.2007.10.024
Shameli, K.; Ahmad, B.M.; Zargar, M.; Yunus, W.M.; Ibrahim, N.A.; Shabanzadeh, P.; Moghaddam, M.G.: Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int. J. Nanomedicine 6, 271–284 (2011). https://doi.org/10.2147/IJN.S16043
Rivera-Garza, M.; Olguín, M.T.; García-Sosa, I.; Alcántara, D.; Rodríguez-Fuentes, G.: Silver supported on natural Mexican zeolite as an antibacterial material. Micropor. Mesopor. Mat. 39, 431–444 (2000). https://doi.org/10.1016/S1387-1811(00)00217-1
Shameli, K.; Ahmad, M.B.; Zargar, M.; Yunus, W.M.; Rustaiyan, A.; Ibrahim, N.A.: Synthesis of silver nanoparticles in montmorillonite and their antibacterial behaviour. Int. J. Nanomedicine 6, 581–590 (2011). https://doi.org/10.2147/IJN.S17112
Krishnan, B.; Mahalingam, S.: Ag/TiO2/bentonite nanocomposite for biological applications: synthesis, characterization, antibacterial and cytotoxic investigations. Adv. Powder Technol. 28, 2265–2280 (2017). https://doi.org/10.1016/j.apt.2017.06.007
Moosa, S.; Mohd Faisol Mahadeven, A.N.; Shameli, K.: Physiochemical synthesis of silver/kaolinite nanocomposites and study their antibacterial properties. J Res. Nanosci. Nanotechnol. 1, 1–11 (2021). https://doi.org/10.37934/jrnn.1.1.111
Guidelines for drinking-water quality: fourth edition incorporating the first addendum. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO. Accessed December 2023.
Acknowledgements
The author would like to acknowledge the Department of Science and Innovation and the Council for Scientific and Industrial Research for financial support. The author also wishes to thank the characterization facility team at the Center for Nanostructured and Advanced Materials.
Funding
Open access funding provided by University of South Africa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Motshekga, S.C. Comparative Antibacterial Activity of Clay-Supported Silver Nanoparticles Prepared by Conventional Heating and Microwave Methods. Arab J Sci Eng (2024). https://doi.org/10.1007/s13369-024-08863-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13369-024-08863-4