Abstract
Laboratory experiments were conducted to determine the thermal tolerance of the living ostracod Cyprideis sp. from a lagoon known as Murray’s Pool on the east coast of Bahrain, in the western Arabian Gulf. Our experimental trials, run in duplicate using a semi-controlled thermal incubator, demonstrate the resilience of the ostracod community to elevated temperatures. We observed that ostracod specimens begin to enter an inactive condition or become comatose at about 39.4 °C, and with increase in temperatures, half of the specimens died or did not recover at 51.8 °C. At 53.5 °C, total mortality is observed with no indication of recovery. These observations have implications for climate change predictions in the western Gulf region, as water temperatures in the lagoon reach 42 °C in summer, while the substrate temperatures on mud flats exposed during low tide exceed the lethal limit of the ostracods during the summer months.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Arabian Gulf faces serious environmental challenges under current conditions of global warming; for example, coral bleaching events are increasing in frequency and intensity [1, 2], which will have cascading consequences for the wider marine communities. A recent climate modeling study [3] predicted that the region between Qatar and Saudi Arabia will be the region most intensely affected by future climate change, with predicted wet bulb air temperatures exceeding 35°C—a lethal temperature for most animals [4, 5].
The Island of Bahrain sits squarely within the area that will be most affected by climate change, and the Kingdom is already the sixth warmest country on the planet with a mean annual air temperature of 29.15 °C (https://tradingeconomics.com/country-list/temperature). Temperatures in Bahrain have risen considerably in the twenty-first century, and according to the Bahrain Meteorological Directorate, the mean monthly temperature for August 2021 was the warmest on record at 36.8 °C. This is already 1.7 °C above the long-term average for the month of August (www.newsofbahrain.com/bahrain/83935.html).
Despite dire predictions for the future state of many ocean ecosystems, including the Arabian Gulf [3, 6,7,8], no experimental studies have been performed on the intertidal marine fauna in the western Gulf to determine their thermal tolerance or lethal limits. A recent study of intertidal and subtidal marine species in Singapore and Thailand has reported upper lethal temperature limits between 41 and 52 °C [9], but such temperatures are already routinely exceeded in Bahrain during summer on intertidal mudflats [10, 11]. In order to refine our predictions about the future of the biocalcifying marine benthos in the Gulf, it is necessary to carry out laboratory experiments to determine the lethal limits of the organisms living in the shallow-marine environments.
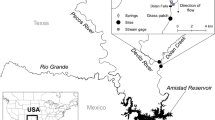
Our research team has been monitoring a small lagoon in eastern Bahrain that experiences thermal stress during the summer months [10, 11]. The locality is known in the scientific literature as ‘Murray’s Pool’ named after the late Professor John Murray (Fig. 1a). Murray’s Pool is located between latitudes 26°02′33"N and 26°02′41"N and longitudes 50°37′20"E and 50°37′31"E and situated precisely at the border between the seaside park in Askar Town and the Marine Fish Hatcheries Facility Center in eastern Bahrain. Until recently, it was the only site in the Kingdom of Bahrain where foraminiferal studies have been carried out [12,13,14]. It is one of the last remaining relatively undisturbed lagoons in eastern Bahrain, with minimal human disturbance and low to undetected anthropogenic pollutants such as trace metals [13, 14]. The lagoon is host to a large variety of marine organisms and wader birds [15]. Occasionally, flood conditions due to periodical high tides can be observed using satellite imagery from Google Earth that records specific times of the day (Fig. 1b). Dark patches in Fig. 1c–f represent wet surfaces that flood during high tide and dry out throughout the day.
Location map of the study area (Murray’s Pool) in eastern shore of Bahrain with different conditions during various times: a simplified map of the locality compared to the surrounding area, b in the current timeline (November 2022) with more detailed features, c during the water-infilling period in winter (December 2014), d, e during the dried-out period in late winter (March 2015 and 2018), and f during the overflooding period in late spring or early summer season (June 2019)
In a survey of macro- and meiofaunal organisms conducted in the summer of 2019 [11], we reported the existence of a ‘dead zone’ in the upper reaches of the intertidal zone in Murray’s Pool, where surficial substrate temperatures exceed 52 °C during summer. Likewise, maximum substrate and water temperatures recorded using in situ temperature monitors can elevate significantly during the day in the summer period within a one-hour interval [11]. In Murray’s Pool, living meiofaunal organisms were only observed in the tidal channels and permanent pools in the summer months where salinity varies between 43 and 47 PSU [13], among which the ostracods are especially abundant. The purpose of study is to determine the thermal tolerances of these ostracods, in order to make more accurate predictions about the future effects of climate change on shallow-water marine organisms in the Gulf region. We are unaware of any comparable modern study of upper thermal limit based on ostracods, regionally in the Middle East and on a global scale.
2 Material and Methods
2.1 Specimen Selection
Specimens of living ostracods were collected from a distal part of Murray’s Pool (farthest pool from the tidal channel inlet; see Fig. 1b) in August 2022 and later additional sampling was conducted in April 2023 for replicate trials. Sediment samples were collected by dragging an open sample jar along the surface of the bottom mud of the pool. Temperatures were recorded in situ using a thermometer probe (TAYLOR RA44069 806GW Digital Thermometer), with an accuracy of 0.1°C. Samples of sediment and water were immediately transferred to the Micropaleontology Laboratory at King Fahd University of Petroleum & Minerals (KFUPM), and ostracods were picked out using fine brush and transferred into a glass petri dish containing the sampled pool water with a temperature around 20.1°C and salinity around 46.5 PSU. Only living ostracods that showed signs of movement were used for the experiment. To verify that no specimen was immobile or dead before the beginning of the experiment, we took composite images of our specimens documenting their pre- and post-experiment positions using a Leica S9i Digital Stereo Microscope, and example of which is given in Fig. 2. All the specimens studied belong to the genus Cyprideis, a single species we designate as Cyprideis sp. due to lack of taxonomical studies of those specimens in our current locality, and the fact that they share certain external morphological features as illustrated by [16].
2.2 Upper Thermal Limit Experiment
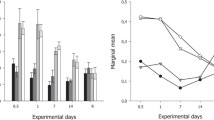
A number of requirements relating to the thermal experiment need to be met in order to produce a proper ecological experiment [17]. These requirements include having knowledge of the initial conditions, sufficient controls within the experiment, and adequate replication. This experiment was conducted in one main exposure and one control session (duplicate in total) at one temperature interval in order to comply with these requirements, with the actual environmental parameters at the field station where the specimens were sampled already specified. The petri dish containing 30 specimens of living Cyprideis sp. was placed in an incubator (Memmert Universal Oven U Incubator I UN 30) initially set at 30 °C. The ostracods’ reactions to heating at specific time intervals and temperature ranges were observed in this study, using different specimens and stages of temperature increase (one set of specimens for each experiment course). In the main experimental scenario, the petri dish containing the ostracods was heated with a 5°C temperature increment and exposed for 10 min before proceeding to the next temperature value, with the first measurement commencing at 30°C air temperature and 10 stages of temperature increase with 5°C increments. In this scenario, the air temperature inside the incubator reaches the targeted level at various intervals until the temperature indicator blinks as representing stabilized internal air temperature, but the air temperature does not represent the water temperature inside the petri dish (which is lower than the air temperature inside the incubator due to water’s heat capacity). After each time interval, the temperature values of the water of each end-to-end of a 10-min time interval were measured using a portable probe thermometer (TAYLOR RA44069 806GW Digital Thermometer), with an accuracy of 0.1°C. Figure 3 presents the air temperature in the incubator and the water temperature in the petri dish in the main and replicate experiments.
2.3 Behavior Observation during Recovery Intersession
At the culmination of each 10-min exposure interval within the incubator experiment, we evaluated the state of our specimens by assessing their mobility (agile or sluggish), as well as their activity level (active or sedentary). Up to five minutes of recovery time were added after observations at room temperature outside the incubator chamber in order to establish the temporary conditions of our specimens before moving on to the next higher temperature exposure while observing their actual condition and behavior using an Olympus SZX7 Stereomicroscope. Due to technical limitations in our study, it was not possible to directly observe when the specimens stop moving or how they behave during heating within the incubator. The immobile specimens were later separated from the moving ones and when none of the test subjects moved after the five-minute recovery period and applying direct stimulus to their body parts (such as their legs) using a needle, the ostracods were assumed to be dead, and the experiment was declared finished. A longer waiting period up to one hour was established for the end-stage experiment to confirm that all of the specimens were dead (when no specimen was observed to move after the final exposure interval). To document the visual death condition, we captured the postmortem image the next day upon termination of the previous experiment, to confirm that the specimens used in the experiment were not simply comatose after one day post-experiment.
In this study, we determined the heat coma temperature (HCT) when at least one specimen started to become immobile at a certain temperature, but later regained consciousness (not total mortality/true death). We also determined the median lethal temperature limit (LT50), corresponding to the temperature at which half of the individuals in the sample are dead after five minutes waiting time, and the upper thermal tolerance (LT100) also known as the upper lethal temperature (ULT), corresponding to the temperature at which total mortality of the sampled population is observed. In addition, we also added median thermal comatose limit (CT50) and upper comatose limit (CT100). No salinity measurements were made during this experiment due to equipment limitation, although we are aware of a possible salinity effect caused by evaporation associated with the elevated temperatures that were achieved during the experimental trials.
3 Results
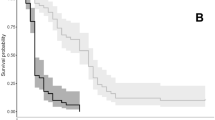
In our investigation, we found the initial thermal effect on the specimens (the HCT) began when at least one specimen entering comatose state (not dead) at 39.3°C; CT50 occurred around 46–47 °C (no record for the 50% comatose status during experiment); and the CT100 was observed at 47.9 °C. Additionally, within our observation, the initial lethal effect on the specimens began around 47.2 °C; LT50 occurs around 51.8 °C, and the ULT was observed around 53.5°C. Survivorship curves of the specimens are shown in Fig. 4. All of the above-mentioned values are based on the average of the main experiment and control, determined by calculating data between main experiment and control experiment.
Within that figure, the number of specimens fluctuates as their mobility is observed and their current status is determined. For example, between 39.3 and 47.1°C, specimens are momentarily immobilized or enter a comatose condition, but later regain consciousness and start to show signs of life during the observation. This is the difference between ‘active/moving’ and ‘living’ status in Fig. 4. On the other hand, above 47.2°C, some specimens were motionless even after the waiting time; hence, their status was changed in the graphs from living to dead. The observed behavior of our specimens during the comatose condition consisted of temporary body part immobility during which their carapace is widely open, and therefore, the animals are not protecting themselves from the short-term environmental stress such as salinity changes, oxygen stress, and aerial exposure with possible heat inducement [18].
4 Discussion
There is currently a significant gap in our knowledge of shallow-marine ostracods from the western Arabian Gulf region. The taxonomy and occurrence of marine ostracods have been studied in the Iranian sector of the northwestern Gulf [19], from Holocene marine sediments cored near Basra in southern Iraq [20], from Kuwait Bay [21], and from the lagoon in Abu Dhabi [22], but so far, no comprehensive studies have been carried out in the area of Bahrain. The presence of living ostracods in Murray’s Pool during summer was previously mentioned, but no details of their taxonomy or specific species determination were given [11]. We identified the ostracod living in Murray’s Pool to be a species belonging to the genus Cyprideis. The species bears some resemblance to Cyprideis torosa (Jones, 1850) (see Fig. 5), such as similar valve (or carapace) and hinge, smooth morphology, and external sensilla that extrude from the carapaces; more or less similar to the specimen illustrated in Mostafawi’s work [19]. Cyprideis torosa is a euryhaline species with a wide geographical distribution, which is likely carried by birds along their migration pathways [23]. The species is globally distributed and can be found in marshes, lakes, lagoons, and bays that are less than 30 m deep and with salinities ranging from 0.1‰ to more than 60‰ [18]. Moreover, the species has been reported in numerous studies performed at a variety of locations, including the Mediterranean, Greece, and the Arabian Gulf [19, 23].
Specimen of Cyprideis sp. from Murray’s Pool, Askar, Bahrain, 1a. Dorsal view; 1b. Right lateral view, 1c. Left lateral view; scale bar = 100 µm; hair-like structure on the images represents the sensilla, similarly on the plate within Schön’s work [16]
In Murray’s Pool, the shallow water of the lagoon can heat up to temperatures in excess of 42 °C during summer, yet this Cyprideis population continues to thrive in the shallow pools. Some ostracod species are known to inhabit extreme environments such as hot springs and hydrothermal vents [24, 25], and we suspected that the Murray’s Pool ostracods might also be pre-adapted to elevated temperatures.
In our investigation, the initial thermal effect on the living individuals began around 39.4°C when specimens become comatose. The first confirmed death occurred around 47.5 °C; half of the specimens died around 51.8°C; and full mortality was observed at 53.5°C. This is the first thermal tolerance experiment of its kind carried out in the Gulf area, and establishes a baseline for explaining the presence of the kill zone observed on the mudflat in Murray’s Pool during summer [11], where afternoon substrate temperatures at low tide exceeded the ULT of the ostracods. Our literature review revealed that experiments testing the thermal tolerance or upper thermal limits of benthic organisms have been only performed on intertidal crustaceans such as crabs and shrimp [26, 27], in addition to meiofauna such as copepods [28], with an overall thermal tolerance below 40°C. In a broader sense, when the other crustaceans have already suffered mortality, the lagoonal ostracods in our experiment (in case of findings within the same area) are just beginning to become impacted by exposure to a water temperature of 40°C based on our experimental works.
We focused on determining the upper lethal limit of ostracods in our experiment because of the environmental variables currently impacting the Arabian Gulf region, notably the warmer sea surface temperatures brought on by human-induced climate change that threaten the intertidal marine communities [6, 29]. Hence, more studies about this ecological aspect between temperature constraints with living communities in the Arabian Gulf specifically need to be further outlined and extensively studied [30]. It is hypothesized that the ostracods found inside the tidal pool are sensitive to variations in water temperature because of the very shallow water and heat from the surrounding mudflats. In the warmest months of the summer, between June and August, the water temperature in the tidal channel and on the pool’s surface can increase beyond 42 °C [11]. Bahrain has already experienced a significant increase in average air temperatures since the mid 1980’s, and temperatures have continued to rise in more recent years [31]. Because of the observed rise in air and water temperatures and projections from climate models, the situation will likely only get worse for the shallow-marine animals living in the western Gulf region. According to published climate models [3, 32], the Arabian Gulf nations’ land surfaces will reach temperatures above 55 °C by the end of the century, while coastal waters surface temperatures could exceed 44 °C. Our ongoing field observations on the Saudi Arabian coastline reveal even higher measured land surface temperatures, as high as 60°C on lagoonal mudflats during the summer period [33]. Based on our observations of ostracods from Murray’s Pool, these temperatures would be lethal for marine organisms that are living within the shallow pools.
5 Conclusions
We conclude that the shallow-water ostracods in Murray’s Pool are experiencing stress from elevated temperatures during summer, when the laboratory-determined coma temperature (starting from 39.4 °C) may be exceeded by several degrees. The LT50 of the Cyprideis population in the pool is determined to be at 51.8°C, and the ULT is in the proximity of 53.5 °C. This finding that shallow-marine organisms are experiencing thermal stress is in line with predictions of climate models, which speculate that portions of the Arabian Gulf will become too hot to sustain eukaryotic life in the next decades [3]. Additional thermal tolerance research on different ostracod species and other calcifying benthic organisms (i.e., gastropods, bivalves, and foraminifera) is needed in order to better understand the thermal limits of the marine fauna in the Arabian Gulf; as well as implementing the experiment with high precision, fully controlled, using a standardized incubator or apparatus that enables us to directly observe their behavior. Considering the possibility of the salinity effect on the specimens, future experiments should address the question of salinity changes during the experimental trials, a topic that was not the main concern of this study.
References
Riegl, B.; Johnston, M.; Purkis, S.; Howells, E.; Burt, J.; Steiner, S.C.C.; Sheppard, C.R.C.; Bauman, A.: Population collapse dynamics in Acropora downingi, an Arabian/Persian Gulf ecosystem-engineering coral, linked to rising temperature. Glob. Chang. Biol. 24, 2447–2462 (2018). https://doi.org/10.1111/gcb.14114
Burt, J.A.; Paparella, F.; Al-Mansoori, N.; Al-Mansoori, A.; Al-Jailani, H.: Causes and consequences of the 2017 coral bleaching event in the southern Persian/Arabian Gulf. Coral Reefs 38, 567–589 (2019). https://doi.org/10.1007/s00338-019-01767-y
Pal, J.S.; Eltahir, E.A.B.: Future temperature in southwest Asia projected to exceed a threshold for human adaptability. Nat. Clim. Chang. 6, 197–200 (2016). https://doi.org/10.1038/nclimate2833
Sunday, J.M.; Bates, A.E.; Dulvy, N.K.: Global analysis of thermal tolerance and latitude in ectotherms. Proceedings of the Royal Society B: Biological Sciences 278(1713), 1823–1830 (2011). https://doi.org/10.1098/rspb.2010.1295
Sunday, J.M.; Bates, A.E.; Dulvy, N.K.: Thermal tolerance and the global redistribution of animals. Nat. Clim. Chang. 2(9), 686–690 (2012). https://doi.org/10.1038/nclimate1539
Wabnitz, C.C.; Lam, V.W.; Reygondeau, G.; Teh, L.C.; Al-Abdulrazzak, D.; Khalfallah, M.; Pauly, D.; Palomares, M.L.D.; Zeller, D.; Cheung, W.W.: Climate change impacts on marine biodiversity, fisheries and society in the Arabian Gulf. PLoS ONE 13(5), 1–26 (2018). https://doi.org/10.1371/journal.pone.0194537
Noori, R.; Tian, F.; Berndtsson, R.; Abbasi, M.R.; Naseh, M.V.; Modabberi, A.; Soltani, A.; Kløve, B.: Recent and future trends in sea surface temperature across the Persian Gulf and Gulf of Oman. PLoS ONE 14(2), 1–19 (2019). https://doi.org/10.1371/journal.pone.0212790
Zittis, G.; Almazroui, M.; Alpert, P.; Ciais, P.; Cramer, W.; Dahdal, Y.; Fnais, M.; Francis, D.; Hadjinicolaou, P.; Howari, F.; Jrrar, A.: Climate change and weather extremes in the Eastern Mediterranean and Middle East. Rev. Geophys. 60(3), 1–48 (2022) https://doi.org/10.1029/2021RG000762
Nguyen, K.D.T.; Morley, S.A.; Lai, C.H.; Clark, M.S.; Tan, K.S.; Bates, A.E.; Peck, L.S.: Upper temperature limits of tropical marine ectotherms: Global warming implications. PLoS ONE 6, 6–13 (2011). https://doi.org/10.1371/journal.pone.0029340
Kaminski, M.A.; Garrison, T.F.: Thermoregulatory behavior in the tropical periwinkle Planaxis sulcatus. Arab. J. Sci. Eng. 45, 4817–4822 (2020). https://doi.org/10.1007/s13369-019-04300-z
Kaminski, M.A.; Amao, A.; Babalola, L.; Khamsin, A.B.; Fiorini, F.; Garrison, A.M.; Gull, H.M.; Johnson, R.L.; Tawabini, B.; Frontalini, F.; Garrison, T.F.: Substrate temperature as a primary control on meiofaunal populations in the intertidal zone: A dead zone attributed to elevated summer temperatures in eastern Bahrain. Reg. Stud. Mar. Sci. 42, 101611 (2021). https://doi.org/10.1016/j.rsma.2021.101611
Basson, P.W.; Murray, J.W.: Temporal variations in four species of intertidal foraminifera, Bahrain. Arabian Gulf. Micropaleontology 41, 69–76 (1995). https://doi.org/10.2307/1485882
Amao, A.O.; Kaminski, M.A.; Setoyama, E.: Diversity of foraminifera in a shallow restricted lagoon in Bahrain. Micropaleontology 62, 197–211 (2016). https://doi.org/10.47894/mpal.62.3.01
Arslan, M.; Kaminski, M.A.; Tawabini, B.S.; Ilyas, M.; Babalola, L.O.; Frontalini, F.: Seasonal variations, environmental parameters, and standing crop assessment of benthic foraminifera in eastern Bahrain. Arabian Gulf. Geological Quarterly 60, 26–37 (2016). https://doi.org/10.7306/gq.1242
Al-Sayed, H.; Naser, H.; Al-Wedaei, K.: Observations on macrobenthic invertebrates and wader bird assemblages in a protected marine mudflat in Bahrain. Aquat. Ecosyst. Health Manage. 11(4), 450–456 (2008). https://doi.org/10.1080/14634980802515948
Schön, I.; Halse, S.; Martens, K.: Cyprideis (Crustacea, Ostracoda) in Australia. J. Micropalaeontol. 36(1), 31–37 (2017). https://doi.org/10.1144/jmpaleo2016-032
Hairston, N.G.: Ecological experiments: purpose, design and execution. Cambridge University Press, Cambridge (1989)
Athersuch, J.; Horne, D.J.; Whittaker, J.E.: Marine and brackish water ostracods (superfamilies Cypridacea and Cytheracea): keys and notes for the identification of the species (Vol. 43). Brill Archive. (1989)
Mostafawi, N.: Recent ostracods from the Persian Gulf. Senckenb. Marit 32, 51–75 (2003). https://doi.org/10.1007/BF03043085
Al-Jumaily, W.A.; Al-Sheikhly, S.S.: Palaeozoogeography of Shallow Marine Ostracoda from Holocene Sediments-Southern Iraq. Qatar Univ. Sci. J, 18, 215–230 (1999)
Al-Abdul-Razzaq, S.; Shublaq, W.; Al-Sheikh, Z.; Kittaneh, W.: Ecology and distribution of ostracods in Kuwait Bay. J Micropalaeontol 2(1), 39–45 (1983)
Bate, R.H.: The distribution of recent ostracoda in the Abu Dhabi Lagoon, Persian Gulf. In Colloquium on the Paleoecology of Ostracodes (1971)
Wouters, K.: On the modern distribution of the euryhaline species Cyprideis torosa (Jones, 1850) (Crustacea, Ostracoda). J Micropalaeontol 36, 21–30 (2017). https://doi.org/10.1144/jmpaleo2015-021
Wickstrom, C.E.; Castenholz, R.W.: Dynamics of cyanobacterial and ostracod interactions in an oregon hot spring. Ecology 66, 1024–1041 (1985). https://doi.org/10.2307/1940563
Karanovic, I.; Brandão, S.N.: Biogeography of deep-sea wood fall, cold seep and hydrothermal vent Ostracoda (Crustacea), with the description of a new family and a taxonomic key to living Cytheroidea. Deep Sea Res. Part II 111, 76–94 (2015). https://doi.org/10.1016/j.dsr2.2014.09.008
Hopkin, R.S.; Qari, S.; Bowler, K.; Hyde, D.; Cuculescu, M.: Seasonal thermal tolerance in marine Crustacea. J. Exp. Mar. Biol. Ecol. 331, 74–81 (2006). https://doi.org/10.1016/j.jembe.2005.10.007
Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.: Thermal tolerance and potential impacts of climate change on coastal and estuarine organisms. J. Sea Res. 70, 32–41 (2012). https://doi.org/10.1016/j.seares.2012.03.002
Sasaki, M.; Dam, H.G.: Global patterns in copepod thermal tolerance. J. Plankton Res. 43, 598–609 (2021). https://doi.org/10.1093/plankt/fbab044
Sheppard, C.; Al-Husiani, M.; Al-Jamali, F.; Al-Yamani, F.; Baldwin, R.; Bishop, J.; Benzoni, F.; Dutrieux, E.; Dulvy, N.K.; Durvasula, S.R.V.; Jones, D.A.: The Gulf: a young sea in decline. Mar. Pollut. Bull. 60(1), 13–38 (2010). https://doi.org/10.1016/j.marpolbul.2009.10.017
Ben-Hasan, A.; Christensen, V.: Vulnerability of the marine ecosystem to climate change impacts in the Arabian Gulf—an urgent need for more research. Global ecology and conservation 17, 1–7 (2019). https://doi.org/10.1016/j.gecco.2019.e00556
Alshehabi, G.: Bahrain News: Bahrain feels the heat from global warming. www.newsofbahrain.com/bahrain/83935.html (2019). Accessed 1 May 2023.
Safieddine, S.; Clerbaux, C.; Clarisse, L.; Whitburn, S.; Eltahir, E.A.B.: Present and future land surface and wet bulb temperatures in the Arabian Peninsula. Environ. Res. Lett. 17, 1–9 (2022). https://doi.org/10.1088/1748-9326/ac507c
Kaminski, M.; Prayudi, S.D.;, Korin, A.; Tawabini, B.S.: Intertidal lagoons in the western Arabian Gulf are summer dead zones (No. EGU23-7210). Copernicus Meetings. (2023) https://doi.org/10.5194/egusphere-egu23-7210
Acknowledgements
We are grateful to the Deanship of Scientific Research, KFUPM, for funding the study through grant DF191042. We also acknowledge laboratory support from the College of Petroleum & Geosciences at KFUPM. We thank David Horne for help with the identification of the ostracod species, and Peter Frenzel, Laura Gemery, and Alan Lord for reviewing the paper and providing helpful comments, in both the formal and informal peer-review processes.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Prayudi, S.D., Korin, A. & Kaminski, M.A. Thermal Tolerance of the Shallow-Water Ostracod Cyprideis from a Lagoon in Bahrain, Western Arabian Gulf. Arab J Sci Eng 49, 121–128 (2024). https://doi.org/10.1007/s13369-023-08570-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-023-08570-6