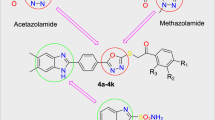
Abstract
The synthetic protocol of our targeted thiadiazole-triazole analogs hybridized with thiophene is based on heterocyclization key compound 5-(2-chloroacetamido)-2-phenyl-N-(1,3,4-thiadiazol-2-yl)-2H-1,2,3-triazole-4-carboxamide (1) in the presence of different thiocarbamoyl reagents 2, 5, and/or 8. The HOMO–LUMO energies and Fukui’s indices were established by applying the DFT/B3LYP molecular modeling methodology. The examined compounds exhibited a low and close HOMO–LUMO energy gap of 1.64–1.99 eV, where the derivatives 7 and 4 presented the lowest and highest values, respectively. The antioxidant properties of the prepared thiadiazole–triazole–thiophene hybrids were investigated using the DPPH radical scavenging technique, where hybrids 4 and 6 showed the strongest inhibition, whereas analog 10 showed only moderate inhibition. Over the course of two common reference drugs, vitamin C and BHT, all of the radical scavenging for thiadiazole-triazole hybrids was examined. Additionally, the generated thiadiazole–triazole–thiophene hybrids' molecular docking was evaluated through the PDB code 3MNG. Hybrid 6 had the highest recorded binding score when compared to the other hybrids. The docking repercussions were appropriate and addressed with the antioxidant assessment.








Similar content being viewed by others
References
Belen’kii, L.I.; Gazieva, G.A.; Evdokimenkova, M.; Soboleva, N.O.: The literature of heterocyclic chemistry. Adv. Heterocycl. Chem. 132, 385–468 (2020). https://doi.org/10.1016/bs.aihch.2020.01.002
Acharya, P.T.; Bhavsar, Z.A.; Jethava, D.J.; Patel, D.B.; Patel, H.D.: A review on development of bio-active thiosemicarbazide derivatives: recent advances. J. Mol. Struct. 1226, 129268 (2021). https://doi.org/10.1016/j.molstruc.2020.129268
Martins, P.; Jesus, J.; Santos, S.; Raposo, L.R.; Roma-Rodrigues, C.; Baptista, B.V.; Fernandes, A.R.: Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules 20(9), 16852–16891 (2015). https://doi.org/10.3390/molecules200916852
Huo, H.; Li, G.; Shi, B.; Li, J.: Recent advances on synthesis and biological activities of C-17 aza-heterocycle derived steroids. Bioorg. Med. Chem. 69, 116882 (2022). https://doi.org/10.1016/j.bmc.2022.116882
Heravi, M.M.; Sadjadi, S.: Recent advances in the application of the Sonogashira method in the synthesis of heterocyclic compounds. Tetrahedron 65(37), 7761–7775 (2009)
Bashir, M.; Bano, A.; Ijaz, A.S.; Chaudhary, B.A.: Recent developments and biological activities of N-substituted carbazole derivatives: a review. Molecules 20(8), 13496–13517 (2015). https://doi.org/10.1016/j.tet.2009.06.028
Zayda, M. G.; Abdel-Rahman, A. A. H.; El-Essawy, F. A.: Synthesis and antibacterial activities of different five-membered heterocyclic rings incorporated with pyridothienopyrimidine. 5 (11), 6163–6168 (2020). https://doi.org/10.1021/acsomega.0c00188.
Plescia, F.; Maggio, B.; Daidone, G.; Raffa, D.: 4-(3H)-quinazolinones N-3 substituted with a five membered heterocycle: a promising scaffold towards bioactive molecules. Eur. J. Med. Chem. 213, 113070 (2021). https://doi.org/10.1016/j.ejmech.2020.113070
Maisuradze, M.; Phalavadishvili, G.; Gakhokidze, Matnadze, M.; Tskhvitaia, S.; Kalandia, E.: Novel diazole/triazole and dibenzothiophene dioxide containing pentacyclic systems with promising biological activities. Int. J. Org. Chem. 7 (01), 34–41 (2017). https://doi.org/10.4236/ijoc.2017.71004.
Tumosienė, I.; Jonuškienė, I.; Kantminienė, Mickevičius, S. V.; Beresnevičius, Z. J.: Synthesis and biological activity of 1,3,4-oxa (thia) diazole,1,2,4-triazole-5-(thio) one and S-substituted derivatives of 3-((2-carboxyethyl) phenylamino) propanoic acid. Res. Chem. Inter. 42 (5), 4459–4477 (2016). https://doi.org/10.1007/s11164-015-2290-0.
Burbuliene, M.M.; Sakociute, V.; Vainilavicius, P.: Synthesis and characterization of new pyrimidine-based 1,3,4-oxa (thia) diazole, 1,2,4-triazole and 4-thiazolidinones. ARKIVOC 2, 281–289 (2009). https://doi.org/10.3998/ark.5550190.0010.c24
Sathish-Kumar, S.; Kavitha, H.P.: Synthesis and biological applications of triazole derivatives–a review. Mini Rev. Org. Chem. 10(1), 40–65 (2013). https://doi.org/10.2174/1570193X11310010004
Soltan, O. M.; Shoman, M. E.; Abdel-Aziz, S. A.; M., Narumi, A.; Konno, H.; Abdel-Aziz, M.: Molecular hybrids: a five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy. Eur. J. Med. Chem. 225, 113768 (2021). https://doi.org/10.1016/j.ejmech.2021.113768.
Da Silva Júnior, E.N.; Jardim, G.A.; Jacob, C.; Dhawa, U.; Ackermann, L.: Synthesis of quinones with highlighted biological applications: a critical update on the strategies towards bioactive compounds with emphasis on lapachones. Eur. J. Med. Chem. 179, 863–915 (2019). https://doi.org/10.1016/j.ejmech.2019.06.056
Aggarwal, R.; Sumran, G.: An insight on medicinal attributes of 1, 2, 4-triazoles. Eur. J. Med. Chem. 205, 112652 (2020). https://doi.org/10.1016/j.ejmech.2020.112652
Malani, A. H.; Makwana, A. H.; Makwana, H. R.: A brief review article: various synthesis and therapeutic importance of 1, 2, 4-triazole and its derivatives. Moroccan J. Chem. 5 (1), 41–58 (2017). https://doi.org/10.48317/imist.prsm/morjchem-v5i1.5959.
Veloso, R.V.; Shamim, A.; Lamarrey, Y.; Stefani, H.A.; Sciani, J.M.: Antioxidant and anti-sickling activity of glucal-based triazoles compounds–an in vitro and in silico study. Bioorg. Chem. 109, 104709 (2021). https://doi.org/10.1016/j.bioorg.2021.104709
Alsaedi, A.M.; Almehmadi, S.J.; Farghaly, T.A.; Harras, M.F.; Khalil, K.D.: VEGFR2 and hepatocellular carcinoma inhibitory activities of trisubstituted triazole derivatives. J. Mol. Struct. 1250, 131832 (2022). https://doi.org/10.1016/j.molstruc.2021.131832
Al-Hussain, S.A.; Farghaly, T.A.; Zaki, M.E.; Abdulwahab, H.G.; Al-Qurashi, N.T.; Muhammad, Z.A.: Discovery of novel indolyl-1,2,4-triazole hybrids as potent vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitors with potential anti-renal cancer activity. Bioorg. Chem. 105, 104330 (2020). https://doi.org/10.1016/j.bioorg.2020.104330
Kabi, A.K.; Gujjarappa, R.; Garg, A.; Roy, A.; Sahoo, A.; Gupta, S.; Malakar, C.C.: Highlights on biological activities of 1,3,4-thiadiazole and indazole derivatives. Tailored Funct. Mater. 15, 99–109 (2022). https://doi.org/10.1007/978-981-19-2572-6_7
Castro, A.; Castaño, T.; Encinas, A.; Porcal, W.; Gil, C.: Advances in the synthesis and recent therapeutic applications of 1,2,4-thiadiazole heterocycles. Bioorg. Med. Chem. 14(5), 1644–1652 (2006). https://doi.org/10.1016/j.bmc.2005.10.012
Shukla, P. K.; Verma, A.; Mishra, P., Significance of nitrogen heterocyclic nuclei in the search of pharmacological active compounds. New Perspective in Agricultural and Human Health; Ed., 100–126 (2017).
Bahmani, Y.; Bahrami, T.; Alabadi, A.: Synthesis, cytotoxicity assessment and molecular docking of N-(5-(substituted-benzylthio)-1,3,4-thiadiazole-2-yl)-2-p-fluorophenylacetamide derivatives as tyrosine kinase inhibitors. Indian J. Pharm. Sci. 81(1), 63–70 (2019). https://doi.org/10.4172/pharmaceutical-sciences.1000480
El-Emam, A.A.; Kumar, E.S.; Janani, K.; Al-Wahaibi, L.H.; Blacque, O.; El-Awady, M.I.; Al-Shaalan, N.H.; Percino, M.J.; Thamotharan, S.: Quantitative assessment of the nature of noncovalent interactions in N-substituted-5-(adamantan-1-yl)-1,3,4-thiadiazole-2-amines: insights from crystallographic and QTAIM analysis. RSC adv. 10(17), 9840–9853 (2020). https://doi.org/10.1039/D0RA00733A
Kerru, N.; Gummidi, L.; Bhaskaruni, S.V.; Maddila, S.N.; Singh, P.; Jonnalagadda, S.B.: A comparison between observed and DFT calculations on structure of 5-(4-chlorophenyl)-2-amino-1,3,4-thiadiazole. Sci. Rep. 9(1), 1–17 (2019). https://doi.org/10.1038/s41598-019-55793-5
Sidat, P. S.; Kasim Jaber, T. M.; Vekariya, S. R.; Mogal, A. M.; Patel, A. M.; Noolvi, M.: Anticancer biological profile of some heterocylic moieties-thiadiazole, benzimidazole, quinazoline, and pyrimidine. Pharmacophore, 13(4), 59–71 (2022). https://doi.org/10.51847/rT6VE6gESu.
Joseph, L.; George, M.; Mathews, P.: A review on various biological activities of 1, 3, 4-thiadiazole derivatives. J. Pharm. Chem. Biol. Sci. 3(1), 329–345 (2015)
Taghizadeh, M.; Javidan, A.; Salmani, A.: Synthesis and docking study on thiadiazolo [3,2-a][1,3]diazepin-8(5H)-one derivatives as selective GABAA agonists. J. Sci. Islamic Rep. Iran 28(1), 13–19 (2017)
Barbosa, F.; Pinto, E.; Kijjo, A.; Pinto, M.; Sousa, E.: Targeting antimicrobial drug resistance with marine natural products. Int. J. Antimicrob. Agents. 56(1), 106005 (2020). https://doi.org/10.1016/j.ijantimicag.2020.106005
Janowska, S.; Paneth, A.; Wujec, M.: Cytotoxic properties of 1,3,4-thiadiazole derivatives—a review. Molecules 25(18), 4309 (2020). https://doi.org/10.3390/molecules25184309
Hoser, A. A.; Kamiński, D. M.; Skrzypek, A.; Matwijczuk, A.; Niewiadomy, A.; Gagoś, M.; Woźniak, K.: Interplay of inter-and intramolecular interactions in crystal structures of 1, 3, 4-thiadiazole resorcinol derivatives. Cryst. Growth Des. 18 (7), 3851–3862 (2018). https://doi.org/10.1021/acs.cgd.8b00077.
Kamboj, S.; Singh, R.: Chromanone-a prerogative therapeutic scaffold: an overview. Arab. J. Sci. Eng. 47, 75–111 (2022). https://doi.org/10.1007/s13369-021-05858-3
Alqahtani, A.M.: Synthesis and biological screening of new thiadiazolopyrimidine-based polycyclic compounds. Sci. Rep. 11(1), 1–15 (2021). https://doi.org/10.1038/s41598-021-95241-x
Mabkhot, Y.N.; Kaal, N.A.; Alterary, S.; Al-Showiman, S.S.; Farghaly, T.A.; Mubarak, M.S.: Antimicrobial activity of thiophene derivatives derived from ethyl (E)-5-(3-(dimethylamino) acryloyl)-4-methyl-2-(phenylamino) thiophene-3-carboxylate. Chem. Cent. J. 11(1), 1–11 (2017). https://doi.org/10.1186/s13065-017-0307-z
Althagafi, I.: Molecular modeling and antioxidant evaluation of new di-2-thienyl ketones festooned with thiazole or pyridine moiety. J. Mol. Struct. 1182, 22–23 (2019). https://doi.org/10.1016/j.molstruc.2021.131287
El-Kousy, S.; Mohareb, R.; Sherif, S.: Heterocyclic synthesis with isothiocyanates: an expeditious synthetic route to polyfunctionally substituted thiophene, pyrazole, oxazole,2,3-dihydrothiazole,2-(pyrazol-4-ylidene) thiazole and 5-(thiazol-2-ylidene) pyrimidine derivatives. J. Chem. Res. Synopses (Print). (8), 312–313 (1993). http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=4869778.
El-Shazly, R.: Spectral, magnetic, thermal and electro-chemical studies on ethyl- alpha-(N-phenylthiocarbamyl) acetoacetate complexes. Chem. Pharm. Bull. 47(11), 1614–1617 (1999). https://doi.org/10.1248/cpb.47.1614
El-Shafei, A.; El-Sayed, A.; El-Saghier, A.: A one-pot synthesis of thiopyrane derivatives from ketene S, S-acetals and α, β-unsaturated nitriles under Ptc conditions. Phosphorus Sulfur. Silicon Relat. Elem. 90(4), 213–218 (1994). https://doi.org/10.1080/10426509408016404
Khalafy, J.; Akbari Dilmaghani, K.; Soltani, L.; Poursattar-Marjani, A.: The synthesis of new 5-aminoisoxazoles by reaction of thiocarbamoylcyanoacetates with hydroxylamine. Chem. Heterocycl. Compd. 44(6), 729–734 (2008). https://doi.org/10.1007/s10593-008-0101-x
Pal, T.: Direct determination of mercury in complex-compounds. Indian Chem. Soc. 62, 561–562 (1985). https://doi.org/10.1007/978-3-642-30942-7
Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.: Gaussian 09, Revision A. 1. Wallingford, CT, USA: Gaussian. (2009).
Becke, A.D.: Perspective on “density-functional thermochemistry III the role of exact exchang e.” Theor. Chem. Acc. 103, 361–363 (2000)
Lee, C.; Yang, W.; Parr, R.G.: Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37(2), 785–789 (1988). https://doi.org/10.1103/PhysRevB.37.785
Perdew, J.P.; Wang, Y.: Pair-distribution function and its coupling-constant average for the spin-polarized electron gas. Phys. Rev. B 46(20), 12947–12954 (1992)
Biovia, D. S.: Materials Studio, (2017) https://doi.org/10.1103/PhysRevB.46.12947.
Delley, B.: Ground-state enthalpies: evaluation of electronic structure approaches with emphasis on the density functional method. J. Phys. Chem. A 110(50), 13632–13639 (2006). https://doi.org/10.1021/jp0653611
Althagafi, I.I.; Gaffer, H.E.: Synthesis, molecular modeling and antioxidant activity of new phenolic bis-azobenzene derivatives. J. Mol. Struct. 1182, 22–30 (2019). https://doi.org/10.1016/j.molstruc.2019.01.030
Abdel-Latif, E.; Amer, F.A.: Synthesis of some 4-arylazo-3-hydroxythiophene disperse dyes for dyeing polyester fabrics. Monatsh. Chem. 139(5), 561–567 (2008). https://doi.org/10.1007/s00706-007-0722-2
Abu-Melha, S.: Molecular modeling and antioxidant activity of newly synthesized 3-hydroxy-2-substituted-thiophene derivatives. J. Mol. Struct. 1250, 131821 (2022). https://doi.org/10.1016/j.molstruc.2021.131821
Abdel-Latif, E.; Keshk, E.M.; Khalil, A.-G.M.; Saeed, A.; Metwally, H.M.: Utilization of thioacetanilides in the synthesis of new 4-(4-acetamidophenylazo)thiophene scaffolds and evaluating their anti-oxidant activity. J. Iran. Chem. Soc. 16(3), 629–637 (2019). https://doi.org/10.1007/s13738-018-1540-7
Orif, M.I.; Abdel-Rhman, M.H.: Synthesis, spectral and structural studies on some new isonicotinic thiosemicarbazide complexes and its biological activity. Polyhedron 98, 162–179 (2015). https://doi.org/10.1016/j.poly.2015.06.021
Suwiński, J.; Świerczek, K.; Glowiak, T.: Nucleophilic amination and ring transformation in 2-methyl-4-nitro-1-phenylimidazole. Tetrahedron Lett. 33(51), 7941–7944 (1992). https://doi.org/10.1016/S0040-4039(00)74784-8
Zhang, L. Y.; Tian, L. J.; Zhang, C. F.: 2-Phenyl-2H-1,2,3-triazole-4-carboxylic acid. Acta Crystallogr. Sect. E Struct. Rep. Online 63 (11), o4415-o4415 (2007). https://doi.org/10.1107/S1600536807051823.
Burnett, M.E.; Johnston, H.M.; Green, K.N.: Structural characterization of the aquaporin inhibitor 2-nicotinamido-1,3,4-thiadiazole. Acta Crystallogr. Sect. C Struct. Chem. 71(12), 1074–1079 (2015). https://doi.org/10.1107/S2053229615021130
Garcia, R.C.; Patel, K.; Day, C.S.; Noftle, R.E.: Synthesis and characterization of complexes formed by transition metal ions with thiophene imides as potential precursors to metal ion uptake and release agents. Inorg. Chim. Acta 453, 268–276 (2016). https://doi.org/10.1016/j.ica.2016.08.016
Sajan, D.; Joseph, L.; Vijayan, N.; Karabacak, M.: Natural bond orbital analysis, electronic structure, non-linear properties and vibrational spectral analysis of L-histidinium bromide monohydrate: a density functional theory. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 81 (1), 85–98 (2011). https://doi.org/10.1016/j.saa.2011.05.052.
Bulat, F.A.; Chamorro, E.; Fuentealba, P.; Toro-Labbe, A.: Condensation of frontier molecular orbital Fukui functions. J. Phys. Chem. A 108(2), 342–349 (2004). https://doi.org/10.1021/jp036416r
Serdaroğlu, G.; Uludag, N.; Colak, N.; Rajkumar, P.: Nitrobenzamido substitution on thiophene-3-carboxylate: electrochemical investigation, antioxidant activity, molecular docking. DFT calculations. J. Mol. Struct. 1271, 134030 (2023). https://doi.org/10.1016/j.molstruc.2022.134030
Koopmans, T.: über Die Zuordnung Von Wellenfunktionen Und Eigenwerten Zu Den Einzelnen Elektronen Eines Atoms. Physica. 1, 104–113 (1934). https://doi.org/10.1016/S0031-8914(34)90011-2
Perdew, J.P.; Parr, R.G.; Levy, M.; Balduz, J.L.: Density-functional theory for fractional particle number: derivative discontinuities of the energy. Phys. Rev. Lett. 49(23), 1691–1694 (1982). https://doi.org/10.1103/physrevlett.49.1691
Janak, J.F.: Proof that in density-functional theory. Phys. Rev. B. 18(12), 7165–7168 (1978). https://doi.org/10.1103/PhysRevB.18.7165
Perdew, J.P.; Levy, M.: Density-functional theory of the energy gap. Phys. Rev. Lett. 51(20), 1884–1887 (1983). https://doi.org/10.1103/PhysRevLett.51.1888
Parr, R.G.; Pearson, R.G.: Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc. 105, 7512–7516 (1983). https://doi.org/10.1021/ja00364a00564
Pearson, R.G.: Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. USA 83, 8440–8441 (1986). https://doi.org/10.1073/pnas.83.22.8440
Parr, R.G.; Szentpaly, L.V.; Liu, S.: Electrophilicity index. J. Am. Chem. Soc. 121, 1922–1924 (1999). https://doi.org/10.1021/ja983494x
Gazquez, J.L.; Cedillo, A.; Vela, A.: Electrodonating and electroaccepting powers. J. Phys. Chem. A 111(10), 1966–1970 (2007). https://doi.org/10.1021/jp065459f
Gomez, B.; Likhanova, N. V.; Domínguez-Aguilar, M. A.; Martínez-Palou, R.; Vela, A.; Gazquez, J. L.: Quantum chemical study of the inhibitive properties of 2-Pyridyl-Azoles. J. Phys. Chem., B 110 (18), 8928–8934(2006). https://doi.org/10.1021/jp057143y
Fukui, K.: Role of frontier orbitals in chemical reactions. Science 218(4574), 747–754 (1982). https://doi.org/10.1126/science.218.4574.747
Mulliken, R.S.: Electronic population analysis on LCAO–MO molecular wave functions. II overlap populations, bond orders, and covalent bond energies. J. Chem. Phys. 23, 1841 (1955). https://doi.org/10.1063/1.1740589
Ghosh, A.; Sarkar, A.; Mitra, P.; Banerji, A.; Banerji, J.; Mandal, S.; Das, M.: Crystal structure and DFT calculations of 3,4-seco-lup-20(29)-en-3-oic acid isolated from Wrightia tinctoria: stacking of supramolecular dimers in the crystal lattice. J. Mol. Struct. 980(1–3), 7–12 (2010). https://doi.org/10.1016/j.molstruc.2010.06.011
Bhagyasree, J. B.; Varghese, H. T.; Panicker, C. Y.; Samuel, J.; Van Alsenoy, C.; Bolelli, K.; Yildiz, I.; Aki, E.: Vibrational spectroscopic (FT-IR, FT-Raman, (1)H NMR and UV) investigations and computational study of 5-nitro-2-(4-nitrobenzyl) benzoxazole. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 102, 99–113 (2013). https://doi.org/10.1016/j.saa.2012.09.032.
Olasunkanmi, L.O.; Obot, I.B.; Ebenso, E.E.: Adsorption and corrosion inhibition properties of N-{n-[1-R-5-(quinoxalin-6-yl)-4,5-dihydropyrazol-3-yl]phenyl} methanesulfonamides on mild steel in 1 M HCl: experimental and theoretical studies. RSC Adv. 6(90), 86782–86797 (2016). https://doi.org/10.1039/C6RA11373G
El Adnani, Z.; Mcharfi, M.; Sfaira, M.; Benzakour, M.; Benjelloun, A.; Touhami, M.E.: DFT theoretical study of 7-R-3methylquinoxalin-2 (1H)-thiones (RH; CH3; Cl) as corrosion inhibitors in hydrochloric acid. Corros. Sci. 68, 223–230 (2013). https://doi.org/10.1016/j.corsci.2012.11.020
Mi, H.; Xiao, G.; Chen, X.: Theoretical evaluation of corrosion inhibition performance of three antipyrine compounds. Comput. Theor. Chem. 1072, 7–14 (2015). https://doi.org/10.1016/j.comptc.2015.08.023
Messali, M.; Larouj, M.; Lgaz, H.; Rezki, N.; Al-Blewi, F.; Aouad, M.; Chaouiki, A.; Salghi, R.; Chung, I.M.: A new schiff base derivative as an effective corrosion inhibitor for mild steel in acidic media: experimental and computer simulations studies. J. Mol. Struct. 1168, 39–48 (2018). https://doi.org/10.1016/j.molstruc.2018.05.018
Roy, R.; Krishnamurti, S.; Geerlings, P.; Pal, S.: Local softness and hardness based reactivity descriptors for predicting intra-and intermolecular reactivity sequences: carbonyl compounds. J. Phys. Chem. A 102(21), 3746–3755 (1998). https://doi.org/10.1021/jp973450v
Roy, R.; de Proft, F.D.; Geerlings, P.: Site of protonation in aniline and substituted anilines in the gas phase: a study via the local hard and soft acids and bases concept. J. Phys. Chem. A 102(35), 7035–7040 (1998). https://doi.org/10.1021/jp9815661
Roy, R.K.; Pal, S.; Hirao, K.: On non-negativity of Fukui function indices. J. Chem. Phys. 110(17), 8236–8245 (1999). https://doi.org/10.1063/1.478792
Molyneux, P.: The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Techno. 26(2), 211–219 (2004)
Güdr, A., Influence of total anthocyanins from bitter melon (Momordica charantia Linn) as antidiabetic and radical scavenging agents. Iran. J. Pharm. Res. 15 (1), 301 (2016). PMCID: PMC4986123.
Li, X.; Chen, B.; Xie, H.; He, Y.; Zhong, D.; Chen, D.: Antioxidant structure–activity relationship analysis of five dihydrochalcones. Molecules 23(5), 1162 (2018). https://doi.org/10.3390/molecules23051162
Abu-Melha, S.: Molecular modeling and docking of new 2-acetamidothiazole-based compounds as antioxidant agents. J. Saudi Chem. Soc. 26(2), 101431 (2022). https://doi.org/10.1016/j.jscs.2022.101431
Nural, Y.; Ozdemir, S.; Yalcin, M.S.; Demir, B.; Atabey, H.; Seferoglu, Z.; Ece, A.: New bis-and tetrakis-1,2,3-triazole derivatives: Synthesis, DNA cleavage, molecular docking, antimicrobial, antioxidant activity and acid dissociation constants. Bioorg. Med. Chem. Lett. 55, 128453 (2022). https://doi.org/10.1016/j.bmcl.2021.128453
Mabkhot, Y.N.; Aldawsari, F.D.; Al-Showiman, S.S.; Barakat, A.; Soliman, S.M.; Choudhary, M.I.; Yousuf, S.; Ben Hadda, T.; Mubarak, M.S.: Synthesis, molecular structure optimization, and cytotoxicity assay of a novel 2-acetyl-3-amino-5-[(2-oxopropyl) sulfanyl]-4-cyanothiophene. Molecules 21(2), 214 (2016). https://doi.org/10.3390/molecules21020214
Hamdi, N.; Slimani, I.; Mansour, L.; Alresheedi, F.; Gürbüz, N.; Özdemir, I.: N-Heterocyclic carbene-palladium-PEPPSI complexes and their catalytic activity in the direct C-H bond activation of heteroarene derivatives with aryl bromides: synthesis, and antimicrobial and antioxidant activities. New J. Chem. 45(45), 21248–21262 (2021). https://doi.org/10.1039/D1NJ04606C
Molvi, K.I.; Mansuri, M.; Sudarsanam, V.; Patel, M.M.; Andrabi, S.M.A.; Haque, N.: Synthesis, anti-inflammatory, analgesic and antioxidant activities of some tetrasubstituted thiophenes. J. Enzyme Inhib. Med. Chem. 23(6), 829–838 (2008). https://doi.org/10.1080/14756360701626082
Acknowledgements
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4350527DSR12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author announces that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bayazeed, A., Althumayri, K., Abu-Melha, S. et al. Synthesis, Molecular Modeling, and Antioxidant Activity of New Thiadiazole-Triazole Analogs Hybridized with Thiophene. Arab J Sci Eng 48, 7553–7570 (2023). https://doi.org/10.1007/s13369-022-07572-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07572-0