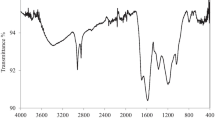
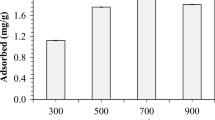
Abstract
Recently, the utilization of peel waste is a significant topic of resource recycling and environment protection. Watermelon is one of the most important fruit species in the world, and its peel accounts for nearly 30% of the weight of watermelon. Here, a series of biochar (WB) derived from watermelon peel and modified biochar (MWB) materials using KOH modifier as adsorbents were successfully prepared with slow pyrolysis at different temperatures (400, 500, 600 and 700 °C). The structure, morphology and specific surface area of the biochar materials were characterized and measured. The optimum adsorption removal conditions of chromium ion (VI) ion from aqueous solution were investigated. The prepared biochar adsorbents had excellent adsorption performance toward Cr(VI) pollutants. The kinetics, isotherm and thermodynamic adsorption process of system were studied. The obtained results indicated that adsorption systems were well followed by pseudo-second-order equation, the Langmuir model (104.17 mg/g of maximum adsorption capacity), endothermic and spontaneous. The recycling experiment demonstrated that the removal efficiency of the optimal material was 85% after 5 cycles. Hence, the prepared modified MWB material is not only helpful to solve the management problem of waste watermelon peel, but also can obtain environment-friendly final green products with potential commercial value.







Similar content being viewed by others
References
Hosseini, S.M.; Moradi, F.; Farahani, S.K.; Bandehali, S.; Parvizian, F.; Ebrahimi, M.; Shen, J.N.: Carbon nanofibers/chitosan nanocomposite thin film for surface modification of poly(ether sulphone) nanofiltration membrane. Mater. Chem. Phys. 269, 124720 (2021)
Wang, C.; Li, X.; Wu, W.; Chen, G.; Tao, J.: Removal of cadmium in water by potassium hydroxide activated biochar produced from Enteromorpha prolifera. J. Water Process Eng. 42, 102201 (2021)
Saravanan, A.; Sundararaman, T.R.; Jeevanantham, S.; Karishma, S.; Kumar, P.S.; Yaashikaa, R.: Effective adsorption of Cu(II) ions on sustainable adsorbent derived from mixed biomass (Aspergillus campestris and agro waste): optimization, isotherm and kinetics study. Groundw. Sustain. Dev. 11, 100460 (2020)
Wu, G.; Liu, Q.; Wang, J.; Zhang, Y.; Yu, C.; Bian, H.; Hegazy, M.; Han, J.; Xing, W.: Facile fabrication of Bi2WO6/biochar composites with enhanced charge carrier separation for photodecomposition of dyes. Colloid Surface A 634, 127945 (2022)
Long, L.; Iqbal, J.; Zhu, Y.; Zhang, P.; Chen, W.; Bhatnagar, A.; Du, Y.: Chitosan/Ag-hydroxyapatite nanocomposite beads as a potential adsorbent for the efficient removal of toxic aquatic pollutants. Int. J. Biol. Macromol. 120, 1752–1759 (2018)
Dong, H.; Zhang, L.; Shao, L.; Wu, Z.; Zhan, P.; Zhou, X.; Chen, J.: Versatile strategy for the preparation of woody biochar with oxygen-rich groups and enhanced porosity for highly efficient Cr(VI) removal. ACS Omega 7, 863–874 (2022)
Wu, G.; Liu, Q.; Wang, J.; Xia, S.; Huang, X.; Han, J.; Xing, W.: Construction of hierarchical Bi2WO6/ZnIn2S4 heterojunction for boosting photocatalytic performance in degradation of organic compounds and reduction of hexavalent chromium. Colloid Surface A 653, 130048 (2022)
Nezamzadeh-Ejhieh, A.; Shahanshahi, M.: Modification of clinoptilolite nano-particles with hexadecylpyridynium bromide surfactant as an active component of Cr(VI) selective electrode. J. Ind. Eng. Chem. 19, 2026–2033 (2013)
Kabir, M.M.; Akter, M.M.; Khandaker, S.; Gilroyed, B.H.; Didar-ul-Alam, Md.; Hakim, M.; Awual, Md.R.: Highly effective agro-waste based functional green adsorbents for toxic chromium (VI) ion removal from wastewater. J. Mol. Liq. 347, 118327 (2022)
Rajapaksha, A.U.; Selvasembian, R.; Ashiq, A.; Gunarathne, V.; Ekanayake, A.; Perera, V.O.; Wijesekera, H.; Mia, S.; Ahmad, M.; Vithanage, M.; Ok, Y.S.: A systematic review on adsorptive removal of hexavalent chromium from aqueous solutions: Recent advances. Sci. Total Environ. 809, 152055 (2022)
Yang, J.; Song, Y.; Yue, Y.; Liu, W.; Che, Q.; Chen, H.; Ma, H.: Chemically dual-modified biochar for the effective removal of Cr (VI) in solution. Polymers 14, 39 (2022)
Foong, C.Y.; Zulkifli, M.F.M.; Wirzal, M.D.H.; Bustam, M.A.; Nor, L.H.M.; Saad, M.S.; Abd Halim, N.S.: COSMO-RS prediction and experimental investigation of amino acid ionic liquid-based deep eutectic solvents for copper removal. J. Mol. Liq. 333, 115884 (2021)
Liu, H.; Li, P.; Zhang, T.; Zhu, Y.; Qiu, F.: Fabrication of recyclable magnetic double-base aerogel with waste bioresource bagasse as the source of fiber for the enhanced removal of chromium ions from aqueous solution. Food Bioprod. Process. 119, 257–267 (2020)
Xing, W.; Liu, Q.; Wang, J.; Xia, S.; Ma, L.; Lu, R.; Zhang, Y.; Huang, Y.; Wu, G.: High selectivity and reusability of biomass-based adsorbent for chloramphenicol removal. Nanomaterials 11, 2950 (2021)
Rajapaksha, A.U.; Alam, M.S.; Chen, N.; Alessi, D.S.; Igalavithana, A.D.; Tsang, D.C.W.; Ok, Y.S.: Removal of hexavalent chromium in aqueous solutions using biochar: chemical and spectroscopic investigations. Sci. Total Environ. 625, 1567–1573 (2018)
Shakya, A.; Agarwal, T.: Removal of Cr(VI) from water using pineapple peel derived biochars: adsorption potential and re-usability assessment. J. Mol. Liq. 293, 111497 (2019)
Pap, S.; Bezanovic, V.; Radonic, J.; Babic, A.; Saric, S.; Adamovic, D.; Sekulic, M.: Synthesis of highly-efficient functionalized biochars from fruit industry waste biomass for the removal of chromium and lead. J. Mol. Liq. 268, 315–325 (2018)
Guo, N.; Lv, X.; Yang, Q.; Xu, X.; Song, H.: Effective removal of hexavalent chromium from aqueous solution by ZnCl2 modified biochar: effects and response sequence of the functional groups. J. Mol. Liq. 334, 116149 (2021)
Peng, Z.; Zhao, H.; Lyu, H.; Wang, L.; Huang, H.; Nan, Q.; Tang, J.: UV modification of biochar for enhanced hexavalent chromium removal from aqueous solution. Environ. Sci. Pollut. Res. 25, 10808–10819 (2018)
Sun, C.; Chen, T.; Huang, Q.; Zhan, M.; Li, X.; Yan, J.: Activation of persulfate by CO2-activated biochar for improved phenolic pollutant degradation: performance and mechanism. Chem. Eng. J. 380, 122519 (2020)
Tayibi, S.; Monlau, F.; Fayoud, N.; Oukarroum, A.; Zeroual, Y.; Hannache, H.; Barakat, A.: One-pot activation and pyrolysis of Moroccan Gelidium sesquipedale red macroalgae residue: production of an efficient adsorbent biochar. Biochar 1, 401–412 (2020)
Wang, L.; Bolan, N.; Tsang, D.; Hou, D.: Green immobilization of toxic metals using alkaline enhanced rice husk biochar: effects of pyrolysis temperature and KOH concentration. Sci. Total Environ. 720, 137584 (2020)
Shi, Y.; Shan, R.; Lu, L.; Yuan, H.; Jiang, H.; Zhang, Y.; Chen, Y.: High-efficiency removal of Cr(VI) by modified biochar derived from glue residue. J. Clean. Prod. 254, 119935 (2020)
Wu, G.; Liu, Q.; Wang, J.; Xia, S.; Wu, H.; Zong, J.; Han, J.; Xing, W.: Facile fabrication of rape straw biomass fiber/β-CD/Fe3O4 as adsorbent for effective removal of ibuprofen. Ind. Crops Prod. 173, 114150 (2021)
Yuan, J.; Zhu, Y.; Wang, J.; Gan, L.; He, M.; Zhang, T.; Li, P.; Qiu, F.: Preparation and application of Mg–Al compositeoxide/coconut shell carbon fiber for effective removal of phosphorus from domestic sewage. Food Bioprod. Process. 126, 293–304 (2021)
Sharma, R.K.; Wooten, J.B.; Baliga, V.L.; Lin, X.; Chan, W.G.; Hajaligol, M.R.: Characterization of chars from pyrolysis of lignin. Fuel 83, 1469–1482 (2004)
Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B.: Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 36, 23–40 (2004)
Chen, B.; Zhou, D.; Zhu, L.: Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. 42, 5137–5143 (2008)
Noiroj, K.; Intarapong, P.; Luengnaruemitchai, A.; Jai-In, S.: A comparative study of KOH/Al2O3 and KOH/NaY catalysts for biodiesel production via transesterification from palm oil. Renew. Energy 34, 1145–1150 (2009)
Liu, Z.G.; Han, G.H.: Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 158, 159–165 (2015)
Nezamzadeh-Ejhieh, A.; Raja, G.: Modification of nanoclinoptilolite zeolite with hexadecyltrimethylammonium surfactant as an active ingredient of chromate-selective membrane electrode. J. Chem. 2013, 1–13 (2012)
Liu, J.; Yang, X.; Liu, H.; Cheng, W.; Bao, Y.: NaOH activation and its adsorption mechanisms for removal of Cu(II) from aqueous solution. Colloid Surface A 601, 124960 (2020)
Yuan, J.; Zhu, Y.; Wang, J.; Liu, Z.; Wu, J.; Zhang, T.; Li, P.; Qiu, F.: Agricultural bamboo leaf waste as carbon precursor for the preparation of Cu-Al/biomass fiber adsorption and its application in the removal of ammonia nitrogen pollutants from domestic wastewater. J. Wood Chem. Technol. 41, 137–149 (2021)
Zhu, Y.; Rong, J.; Mao, K.; Yang, D.; Zhang, T.; Qiu, F.; Pan, J.; Pu, Z.: Boronate affinity-modified magnetic β-cyclodextrin polymer for selective separation and adsorption of shikimic acid. J. Mater. Sci. 56, 13043–13055 (2021)
Yuan, J.; Zhu, Y.; Wang, J.; Liu, Z.; He, M.; Zhang, T.; Li, P.; Qiu, F.: Facile modification of biochar derived from agricultural straw waste with effective adsorption and removal of phosphorus from domestic sewage. J. Inorg. Organom. Polym. Mater. 31, 3867–3879 (2021)
Zhu, Y.; Wang, K.; Lu, J.; Pan, Z.; Rong, J.; Zhang, T.; Yang, D.; Pan, J.; Qiu, F.: Teamed boronate affinity-functionalized Zn-MOF/PAN-derived molecularly imprinted hollow carbon electrospinning nanofibers for selective adsorption of shikimic acid. ACS Appl. Mater. Interface 14, 27294–27308 (2022)
Liu, L.; Liu, X.; Wang, D.; Lin, H.; Huang, L.: Removal and reduction of Cr(VI) in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge. J. Clean. Prod. 254, 120562 (2020)
Zhang, H.; Xiao, R.; Li, R.; Ali, A.; Chen, A.; Zhang, Z.: Enhanced aqueous Cr(VI) removal using chitosan-modified magnetic biochars derived from bamboo residues. Chemosphere 261, 127694 (2020)
Ding, W.; Peng, W.; Zeng, X.; Tian, X.: Effects of phosphorus concentration on Cr(VI) sorption onto phosphorus-rich sludge biochar. Front. Environ. Sci. Eng. 8, 379–385 (2014)
Liang, J.; Chen, Y.; Cai, M.; Gan, M.; Zhu, J.: One-pot pyrolysis of metal-embedded biochar derived from invasive plant for efficient Cr(VI) removal. J. Environ. Chem. Eng. 9, 105714 (2021)
Li, F.; Zimmerman, A.R.; Hu, X.; Gao, B.: Removal of aqueous Cr(VI) by Zn- and Al-modified hydrochar. Chemosphere 260, 127610 (2020)
Khushk, S.; Zhang, L.; Pirzada, A.M.; Irfan, M.; Li, A.: Cr(VI) heavy metal adsorption from aqueous solution by KOH treated hydrochar derived from agricultural wastes. AIP Conf Proc 2119, 020003 (2019)
Zhang, X.; Zhang, L.; Li, A.: Eucalyptus sawdust derived biochar generated by combining the hydrothermal carbonization and low concentration KOH modification for hexavalent chromium removal. J. Environ. Manag. 206, 989–998 (2018)
Thangagiri, B.; Sakthivel, A.; Jeyasubramanian, K.; Seenivasan, S.; Raja, J.D.; Yun, K.: Removal of hexavalent chromium by biochar derived from Azadirachta indica leaves: batch and column studies. Chemosphere 286, 131598 (2020)
Mishra, A.; Gupta, B.; Kumar, N.; Singh, R.; Varma, A.; Thakur, I.S.: Synthesis of calcite-based bio-composite biochar for enhanced biosorption and detoxification of chromium Cr (VI) by Zhihengliuella sp. ISTPL4. Bioresour. Technol. 307, 123262 (2020)
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (21878132).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yuan, Z., Sun, X., Hua, J. et al. Upcycling Watermelon Peel Waste into a Sustainable Environment-Friendly Biochar for Assessment of Effective Adsorption Property. Arab J Sci Eng 48, 9035–9045 (2023). https://doi.org/10.1007/s13369-022-07397-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07397-x