Abstract
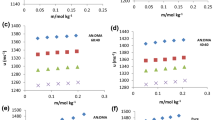
This work presents the investigation of ultrasonic speed propagation waves and aqueous solutions densities of acetates and sulfates of single and double- charged cations in a wide range of salt concentration. The variation of the relative adiabatic compressibility of the solution on the salt concentration has two or several straight lines. For each salt at a certain concentration (Cp), the transition from one straight line to another is occurred. Each of these lines corresponds to a certain value of the adiabatic compressibility of water. It is shown that presence of ions decreases the adiabatic compressibility of water. The hydrate numbers and sizes of nanoparticles (hydrated ions) are estimated as a function of the salt concentration. The obtained results show that the number of hydration decreases stepwise with increasing salt concentration, i.e., in certain intervals the salt concentration remains constant. The evaluated hydrate numbers and radii of hydrated ions (nanoparticle sizes) by the acoustic method are in a good agreement with the literature data.


Similar content being viewed by others
Data Availability
All data are included within the manuscript.
Code Availability
Not applicable.
References
Lee, L.L.: Molecular thermodynamics of electrolyte solutions. World Sci. (2021). https://doi.org/10.1142/6836
Samoilov, O.V.: The structure of aqueous solutions of electrolytes and hydration of ions (translated from Russian). " Izd. Akad. Nauk SSSR, Moscow (1957)
Izutsu, K.: Electrochemistry in nonaqueous solutions translated from Russian, p. 48–92. John Wiley & Sons, US (2009)
Chizhik, V. I. (eds.): NMR-relaxation (translated from Russian), p.154-169. St.-Peterburg (2004)
Smirnov, P.; Trostin, V.: Structural parameters of the nearest environment of ions in aqueous solutions of inorganic electrolytes. JSC Publish. House 67(1), 23–27 (2011). https://doi.org/10.1134/S1070363213010039
Afanasyev, V.N.; Ustinov, N.: Verification of electrolyte solvation from diluted to concentrated in aqueous solutions (translated from Russian). Bull. Moscow State Univ. 52, 323–340 (2021)
Nizomov, Z.A.; Badalov, A.; Mirzoeva, Sh.: Estimation of the hydration numbers of doubly charged cations in aqueous solutions of nitrate salts from ultrasonic data (translated from Russian). Rep. Acad. Sci. Republic of Tajikistan 46, 76–78 (2013)
Burakowski, A.; Gliński, J.: Hydration of urea and its derivatives from acoustic and volumetric methods. Chem. Phys. Lett. 641, 40–43 (2015). https://doi.org/10.1016/j.cplett.2015.10.052
Dilip, H.; Barge, S.: Hydrogen bonding in liquid water and in the hydration shell of salts. ChemPhysChem 17, 902–912 (2016). https://doi.org/10.1002/cphc.201500921
Huang, X.; Cao, Z.; Wang, Q.: The universal characteristic water content of aqueous solutions. Chinese Phys. B 28, 065101 (2019). https://doi.org/10.1088/1674-1056/28/6/065101
Vo, Q.; Bay, M.; Nam, P.; Quang, D.; Flavel, M.; Hoa, N.; Mechler, A.: Theoretical and experimental studies of the antioxidant and antinitrosant activity of syringic acid. J. Org. Chem. 85, 15514–15520 (2020). https://doi.org/10.1021/acs.joc.0c02258
Traustason, H.: Prediction of solution behavior via calorimetric measurements allows for detailed elucidation of polyoxometalate transformation. Inorg. Chem. 60, 6753–6763 (2021). https://doi.org/10.1021/acs.inorgchem.1c00587
Kaatze, U.: Aspects of Ion hydration. adiabatic compressibility compared to the dielectric properties of aqueous electrolyte solutions. J. Phys. Chem. B 117, 12252–12260 (2013). https://doi.org/10.1021/jp407633c
Krakowiak, J.; Wawer, J.; Panuszko, A.: Densimetric and ultrasonic characterization of urea and its derivatives in water. J. Chem. Thermodynam. 58, 211–220 (2013)
Afanasiev, V.N.; Ustinov, A.N.: Solvation of the electrolytic component of Seawater. J. Solution Chem. 36, 853–868 (2007). https://doi.org/10.1007/s10953-007-9152-3
Mikhailov, I.; Soloviev, V.; Syrnikov, Yu.: Fundamentals of Molecular Acoustics. Nauka, Moscow (1964)
Nizomov, Z.; Salnikova, A.: Interparticle interactions in aqueous solutions of sulfates according to IR – spectroscopy (translated from Russian). Rep. Acad. Sci. Republic of Tajikistan 44, 74–78 (2021)
Sarkisov, G.N.: Structural models of water (translated from Russian). Uspekhi fizicheskikh nauk 176, 833–845 (2016)
Antonchenko, V.; Davydov, A.; Ilyin, V. (eds.). Fundamentals of Water Physics (translated from Russian), p.600-667. Kiev: Nauk. Dumka (1991)
Henke, D.E.: A history of ordinary and extraordinary means. Natl Catholic Bioethics Q 5(3), 555–575 (2005). https://doi.org/10.5840/ncbq20055333
Malenkov, G.G.: Structure and dynamics of liquid water. J. Struct. Chem. 47, 5–35 (2006). https://doi.org/10.1007/s10947-006-0375-8
Lyashchenko, A.K.; Dunyashev, L.V.; Dunyashev, V.S.: Spatial structure of water in the entire area of the close order. J. Struct. Chem. 47, 36–53 (2006)
Wiggins, P.M.: Role of water in some biological processes. Microbiol. Rev. 54(4), 432–449 (1990). https://doi.org/10.1128/mr.54.4.432-449.1990
Samoilov, OYa.: To the fundamentals of the kinetic theory of hydrophobic hydration in dilute aqueous solutions (translated from Russian). J. Phys. Chem. 52, 1857–1862 (1978)
Marcus, Y.: Effect of ions on the structure of water: structure making and breaking. Chem. Rev. 109(3), 1346–1370 (2019). https://doi.org/10.1021/cr8003828
Grechishkina, AYu.; Kazimirov, V.P.; Goncharuk, V.V.: Influence of the nature and geometry of ions on the structure of the solvent in aqueous solutions of electrolytes (translated from Russian). J. Chem. Technol. Water 29, 413–421 (2007)
Grossfield, A.: Dependence of ion hydration on the sign of the ions charge. J. Chem. Phys. 122(2), 024506 (2005). https://doi.org/10.1063/1.1829036
Ollis, D.F.: Kinetics of liquid phase photocatalyzed reactions: an illuminating approach. J. Phys. Chem. B 109(6), 2439–2444 (2005). https://doi.org/10.1021/jp040236f
Feliu, S.: Electrochemical impedance spectroscopy for the measurement of the corrosion rate of magnesium alloys: brief review and challenges. Metals 10(6), 775 (2020). https://doi.org/10.3390/met10060775
Acknowledgements
The authors express their gratitude to the leadership of the Research Institute of the Tajik National University for the opportunity to conduct this study in the laboratory of condensed matter physics and molecular spectroscopy.
Funding
No funding to declare. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflicts of interest/competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nizomov, Z., Asozoda, M. & Nematov, D. Characteristics of Nanoparticles in Aqueous Solutions of Acetates and Sulfates of Single and Doubly Charged Cations. Arab J Sci Eng 48, 867–873 (2023). https://doi.org/10.1007/s13369-022-07128-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-07128-2