Abstract
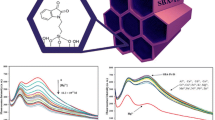
A highly ordered mesoporous silica SBA-15 was modified by indole-3-carbaldehyde followed by covalent grafting of malononitrile to prepare organic–inorganic hybrid nanomaterial (SBA-IC-MN). The characterization of the product presented the successful attachment of organic moieties at the surface of SBA-15 and the preservation of the original structure of SBA-15. The optical sensing ability of SBA-IC-MN was examined via different metal ions in H2O medium by fluorescence spectroscopy. SBA-IC-MN can be considered as highly selective and sensitive toward Hg2+ ions with a detection limit of 3.26 × 10–6. Further, excellent linearity was obtained between the fluorescence intensity of SBA-IC-MN and the concentration of Hg2+ ion.










Similar content being viewed by others
References
Dhakshinamoorthy, A.; Li, Z.; Garcia, H.: Catalylysis and photocatalysis by metal organic framework. Chem. Soc. Rev. 47, 8134–8172 (2018)
Dhakshinamoorthy, A.; Asiri, A.M.; Garcia, H.: 2D metal-organic frameworks as multifunctional materials in heterogeneous catalysis and electro/photocatalysis. Adv. Mater. 31, 1900617 (2019)
Dhakshinamoorthy, A.; Santiago-Portillo, A.; Asiri, A.M.; Garcia, H.: Engineering UiO-66 metal organic framework for heterogeneous catalysis. Chem. Cat. Chem. 11, 899–92399 (2019)
Vojoudi, H.; Badiei, A.; Bahar, S.; Mohammadi Ziarani, G.; Faridbod, F.; Ganjali, M.: Post-modification of nanoporous silica type SBA-15 by bis (3-triethoxysilylpropyl) etrasulfide as an efficient adsorbent for arsenic removal. Powder Technol. 319, 271–278 (2017)
Zhao, L.; Li, J.; Sui, D.; Wang, Y.: Highly selective fluorescence chemosensors based on functionalized SBA-15 for detection of Ag+ in aqueous media. Sens. Actuators 242, 1043–1049 (2017)
Mohajer, F.; Mohammadi Ziarani, G.; Badiei, A.: Decorated palladium nanoparticles on mesoporous organosilicate as an efficient catalyst for Sonogashira coupling reaction. J. Iran. Chem. Soc. 18, 589–601 (2021)
Mohajer, F.; Mohammadi Ziarani, G.; Badiei, A.: The synthesis of SBA-Pr-3AP@ Pd and its application as a highly dynamic, eco-friendly heterogeneous catalyst for Suzuki-Miyaura cross-coupling reaction. Res. Chem. Intermed. 46, 4909–4922 (2020)
Saleh, S.N.M.; Yusoff, M.H.M.; Abdullah, A.Z.: Caesium salt of tungstophosphoric acid supported on mesoporous SBA-15 catalyst for selective esterification of lauric acid with glycerol to monolaurin. Arab. J. Sci. Eng. 43, 5771–5783 (2018)
Şimşek, V.: Investigation of catalytic sustainability of silica-based mesoporous acidic catalysts and ion-exchange resins in methyl acetate synthesis and characterizations of synthesized catalysts. Arab. J. Sci. Eng. 44, 5301–5310 (2019)
Mohammadi Ziarani, G.; Gholamzadeh, P.; Badiei, A.; Fathi Vavsari, V.: The role of pyruvic acid as starting material in some organic reactions in the presence of SBA-Pr-SO3H nanocatalys. Res. Chem. Intermed. 44, 277 (2018)
Hashemi, P.; Shamizadeh, M.; Badiei, A.; ZarabadiPoor, P.; Ghiasvand, A.R.; Yarahmadi, A.: Amino ethyl-functionalized nanoporous silica as a novel fiber coating for solid-phase microextraction. Anal. Chim. Acta. 646, 1–5 (2009)
Yang, P.; Gai, S.; Lin, J.: Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 41, 3679–3698 (2012)
Bahrami, Z.; Badiei, A.; Atyabi, F.: Surface functionalization of SBA-15 nanorods for anticancer drug delivery. Chem. Eng. Res. Des. 92, 1296–1303 (2014)
Fathi Vavsari, V.; Mohammadi Ziarani, G.; Badiei, A.: The role of SBA-15 in drug delivery. RSC Adv. 5, 91686–91707 (2015)
Dashtian, K.; Zare-Dorabei, R.: An easily organic–inorganic hybrid optical sensor based on dithizone impregnation on mesoporous SBA-15 for simultaneous detection and removal of Pb (II) ions from water samples: Response-surface methodology. Appl. Organomet. Chem. 31, e3842 (2017)
Zhao, L.; Sui, D.; Wang, Y.: Fluorescence chemosensors based on functionalized SBA-15 for detection of Pb 2+ in aqueous media. RSC Adv. 5, 16611–16617 (2015)
Dong, Z.; Tian, X.; Chen, Y.; Hou, J.; Ma, J.: Rhodamine group modified SBA-15 fluorescent sensor for highly selective detection of Hg 2+ and its application as an INHIBIT logic devic. RSC Adv. 3, 2227–2233 (2013)
Wang, Y.; Li, B.; Zhang, L.; Liu, L.; Zuo, Q.; Li, P.: A highly selective regenerable optical sensor for detection of mercury (II) ion in water using organic–inorganic hybrid nanomaterials containing pyrene. New J. Chem. 34, 1946–1953 (2010)
Stein, A.; Melde, B.J.; Schroden, R.C.: Hybrid inorganic–organic mesoporous silicates—nanoscopic reactors coming of age. Adv. Mater. 12, 1403–1419 (2000)
Lashgari, N.; Badiei, A.; Ziarani, G.M.; Faridbod, F.: Isatin functionalized nanoporous SBA-15 as a selective fluorescent probe for the detection of Hg (II) in water. Anal. Bioanal. Chem. 409, 3175–3185 (2017)
Karimi, M.; Badieia, A.; Ziarani, G.M.: Fluorescence-enhanced optical sensor for detection of Al3+ in water based on functionalised nanoporous silica type SBA-15. Chem. Paper 70, 1431–1438 (2016)
Wang, Y.; Li, B.; Zhang, L.; Song, H.: Multifunctional mesoporous nanocomposites with magnetic, optical, and sensing features: synthesis, characterization, and their oxygen-sensing performance. Langmuir 29, 1273–1279 (2013)
Leng, B.; Jiang, J.; Tian, H.: A mesoporous silica supported Hg2+ chemodosimeter. AIChE J. 56, 2957–2964 (2010)
De Silva, A.P.; Gunaratne, H.N.; Gunnlaugsson, T.; Huxley, A.J.; McCoy, C.P.; Rademacher, J.T.; Rice, T.E.: Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97, 1515–1566 (1997)
Aragay, G.; Pons, J.; Merkoçi, A.: Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 111, 3433–3458 (2011)
Quang, D.T.; Kim, J.S.: Fluoro-and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 110, 6280–6301 (2010)
Bargossi, C.; Fiorini, M.C.; Montalti, M.; Prodi, L.; Zaccheroni, N.: Recent developments in transition metal ion detection by luminescent chemosensors. Coord. Chem. Rev. 208, 17–32 (2000)
Benoit, J.; Fitzgerald, W.; Damman, A.: The biogeochemistry of an ombrotrophic bog: evaluation of use as an archive of atmospheric mercury deposition. Environ. Res. 78, 118–133 (1998)
Tchounwou, P.B.; Ayensu, W.K.; Ninashvili, N.; Sutton, D.: Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 18, 149–175 (2003)
Von Burg, R.: Inorganic mercury. Appl. Toxicol. 15, 483–493 (1995)
Grandjean, P.; Weihe, P.; White, R.F.; Debes, F.: Cognitive performance of children prenatally exposed to “safe” levels of methylmercury. Environ. Res. 77, 165–172 (1998)
Harada, M.: Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 25, 1–24 (1995)
Renzoni, A.; Zino, F.; Franchi, E.: Mercury levels along the food chain and risk for exposed populations. Environ. Res. 77, 68–72 (1998)
Hoyle, I.; Handy, R.: Dose-dependent inorganic mercury absorption by isolated perfused intestine of rainbow trout, Oncorhynchus mykiss, involves both amiloride-sensitive and energy-dependent pathways. Aquat. Toxicol. 72, 147–159 (2005)
Li, Y.; Yang, L.-L.; Liu, K.; Zhao, F.-Y.; Liu, H.; Ruan, W.-J.: Two hexaazatriphenylene-pyrene based Hg2+ fluorescent chemosensors applicable for test paper detection. New J. Chem. 39, 2429–2432 (2015)
Choi, J.-Y.; Kim, W.-T.; Yoon, J.-Y.: Rhodamine based fluorescent chemosensors for Hg2+ and its biological application. Bull. Korean Chem. Soc. 33, 2359–2364 (2012)
Hu, J.; Li, J.; Qi, J.; Chen, J.: Highly selective and effective mercury (II) fluorescent sensors. New J. Chem. 39, 843–848 (2015)
Gao, B.; Gong, W.-T.; Zhang, Q.-L.; Ye, J.-W.; Ning, G.-L.: A selective “turn-on” fluorescent sensor for Hg2+ based on “reactive” 7-hydroxycoumarin compound. Sens. Actuators B Chem. 162, 391–395 (2012)
So, H.-S.; Rao, B.A.; Hwang, J.; Yesudas, K.; Son, Y.-A.: Synthesis of novel squaraine–bis (rhodamine-6G): A fluorescent chemosensor for the selective detection of Hg2+. Sens. Actuators B Chem. 202, 779–787 (2014)
Shi, L.; Song, W.; Li, Y.; Li, D.-W.; Swanick, K.N.; Ding, Z.; Long, Y.-T.: A multi-channel sensor based on 8-hydroxyquinoline ferrocenoate for probing Hg (II) ion. Talanta 84, 900–904 (2011)
Karimi, M.; Badiei, A.; Mohammadi Ziarani, G.: A single hybrid optical sensor based on nanoporous silica type SBA-15 for detection of Pb2+ and I− in aqueous media. RSC Adv. 5, 36530–36539 (2015)
Karimi, M.; Badiei, A.; Lashgari, N.; Mohammadi Ziarani, G.: A chromotropic acid modified SBA-15 as a highly sensitive fluorescent probe for determination of Fe3+ and I− ions in water. J. Porous Mater. 25, 137–146 (2018)
Lashgari, N.; Badiei, A.; Mohammadi Ziarani, G.: Selective detection of Hg2+ ion in aqueous medium with the use of 3-(pyrimidin-2-ylimino) indolin-2-one-functionalized SBA-15. Appl. Organomet. Chem. 32, e3991 (2018)
Afshani, J.; Badiei, A.; Karimi, M.; Lashgari, N.; Mohammadi Ziarani, G.: A Schiff base-grafted nanoporous silica material as a reversible optical probe for Hg2+ ion in water. Appl. Organomet. Chem. 31, e3856 (2017)
Karimi, M.; Badiei, A.; Ziarani, G.M.: A click-derived dual organic-inorganic hybrid optical sensor based on SBA-15 for selective recognition of Zn2+ and CN− in water. Inorg. Chim. Acta 450, 346–352 (2016)
Lashgari, N.; Badiei, A.; Mohammadi Ziarani, G.: A novel functionalized nanoporous SBA-15 as a selective fluorescent sensor for the detection of multianalytes (Fe3+ and Cr2O72−) in water. J. Phys. Chem. Solids 103, 238–248 (2017)
Afshani, J.; Badiei, A.; Lashgari, N.; Ziarani, G.M.: A simple nanoporous silica-based dual mode optical sensor for detection of multiple analytes (Fe3+, Al3+ and CN−) in water mimicking XOR logic gate. RSC Adv. 6, 5957–5964 (2016)
Newkirk, A.E.: Thermogravimetric measurements. Anal. Chem. 32, 1558–1563 (1960)
Kaushik, R.; Singh, A.; Ghosh, A.: Selective colorimetric sensor for the detection of Hg2+ and H2S in aqueous medium and waste water samples. Chem. Sel 1, 1533–1540 (2016)
Su, Q.; Niu, Q.; Sun, T.; Li, T.: A simple fluorescence turn-on chemosensor based on Schiff-base for Hg2+-selective detection. Tetrahedron Lett. 57, 4297–4301 (2016)
Jiménez-Sánchez, A.; Farfán, N.; Santillan, R.: A reversible fluorescent–colorimetric Schiff base sensor for Hg2+ ion. Tetrahedron Lett. 54, 5279–5283 (2013)
Weng, J.; Mei, Q.; Ling, Q.; Fan, Q.; Huang, W.: A new colorimetric and fluorescent ratiometric sensor for Hg2+ based on 4-pyren-1-yl-pyrimidine. Tetrahedron 68, 3129–3134 (2012)
Wu, D.; Wang, Z.; Wu, G.; Huang, W.: Chemosensory rhodamine-immobilized mesoporous silica material for extracting mercury ion in water with improved sensitivity. Mater. Chem. Phys. 137, 428–433 (2012)
Acknowledgements
The authors thank the research support council of the Alzahra University and the University of Tehran.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interest
The authors declare that there is no declaration of competing interest in this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi Ziarani, G., Ebrahimi, Z., Mohajer, F. et al. A Fluorescent Chemosensor Based on Functionalized Nanoporous Silica (SBA-15 SBA-IC-MN) for Detection of Hg2+ in Aqueous Media. Arab J Sci Eng 47, 397–406 (2022). https://doi.org/10.1007/s13369-021-05518-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05518-6