Abstract
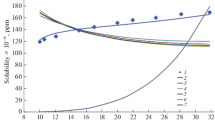
Solubility parameters of supercritical water and methanol were probed by using molecular dynamics simulation. The computed solubility parameters agree well with the theoretical values for different temperatures and pressures within the supercritical region. The results show that the solubility parameter decreases with increasing temperature and increases with increasing the pressure. The polarity of the system increases at higher temperatures; therefore, the degree of molecular aggregation increases. Raising the pressure of the system reduces the degree of aggregation between molecules and increases the solubility parameter of the system.






Similar content being viewed by others
References
Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič, M.; Knez Hrnčič, M.; Škerget, M.: Industrial applications of supercritical fluids: a review. Energy 77, 235–243 (2014). https://doi.org/10.1016/j.energy.2014.07.044
Kiran, E.: Supercritical fluids and polymers—the year in review—2014. J. Supercrit. Fluids 110, 126–153 (2016). https://doi.org/10.1016/j.supflu.2015.11.011
Khaw, K.Y.; Parat, M.O.; Shaw, P.N.; Falconer, J.R.: Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: a review. Molecules (2017). https://doi.org/10.3390/molecules22071186
Knez, Ž.; Cör, D.; Knez, Hrnčič M.: Solubility of solids in sub- and supercritical fluids: a review 2010–2017. J. Chem. Eng. Data 63, 860–884 (2018). https://doi.org/10.1021/acs.jced.7b00778
Chen, L.; Global, I.; Iwamoto, Y.: Advanced Applications of Supercritical Fluids in Energy Systems, p. 2017. Engineering Science Reference, Hershey (2017)
Kruse, A.: Hydrothermal biomass gasification. J. Supercrit. Fluids 47, 391–399 (2009). https://doi.org/10.1016/j.supflu.2008.10.009
Brunner, G.: Near and supercritical water. Part II: oxidative processes. J. Supercrit. Fluids 47, 382–390 (2009). https://doi.org/10.1021/acs.energyfuels.8b01852
Alasiri, H.; Klein, M.T.: A density functional theory probe of the hydrolysis of heavy hydrocarbon structural moieties in supercritical water. Energy Fuels 32, 8700–8704 (2018). https://doi.org/10.1021/acs.energyfuels.8b01852
Jazzar, S.; Quesada-medina, J.; Olivares-carrillo, P.; Néjib, M.; Acién-fernández, F.G.; Fernández-sevilla, J.M.; et al.: Whole biodiesel conversion process combining isolation, cultivation and in situ supercritical methanol transesterification of native microalgae. Bioresour. Technol. 190, 281–288 (2015). https://doi.org/10.1016/j.biortech.2015.04.097
Manuale, D.L.; Torres, G.C.; Vera, C.R.; Yori, J.C.: Study of an energy-integrated biodiesel production process using supercritical methanol and a low-cost feedstock. Fuel Process. Technol. 140, 252–261 (2015). https://doi.org/10.1016/j.fuproc.2015.08.026
Wang, B.; Yang, C.; Xu, J.; Zeng, D.; Jin, T.; Fang, T.: Research on transesterification parameters for producing plant sterols with supercritical methanol. Toxicol. Environ. Chem. 97, 314–325 (2015). https://doi.org/10.1080/02772248.2015.1050184
Sun, Z.; Bottari, G.; Barta, K.: Supercritical methanol as solvent and carbon source in the catalytic conversion of 1,2-diaminobenzenes and 2-nitroanilines to benzimidazoles. Green Chem. 17, 5172–5181 (2015)
Lim, S.; Lee, K.T.: Bioresource technology optimization of supercritical methanol reactive extraction by response surface methodology and product characterization from Jatropha curcas L. seeds. Bioresour. Technol. 142, 121–130 (2013). https://doi.org/10.1016/j.biortech.2013.05.010
Zhang, C.; Xu, L.; Zhang, H.; Yang, J.; Du, J.; Liu, Z.: Determination of solid products from the de-polymerization of poly (trimethylene terephthalate) in supercritical methanol. J. Chromatogr. A 1055, 115–121 (2004). https://doi.org/10.1016/j.chroma.2004.08.146
Hansen, C.M.: Hansen Solubility Parameters: A User’s Handbook, vol. 53, 2nd edn. Taylor & Francis Group, London (2007). https://doi.org/10.1017/cbo9781107415324.004
Kitak, T.; Dumičič, A.; Planinšek, O.; Šibanc, R.; Srčič, S.; Rades, T.; et al.: Determination of solubility parameters of ibuprofen and ibuprofen lysinate. Molecules 20, 21549–21568 (2015). https://doi.org/10.3390/molecules201219777
King, J.W.: Determination of the solubility parameter of soybean oil by inverse gas chromatography. Leb Und-Technologie (Food Sci. Technol.) 28, 190–195 (1995). https://doi.org/10.1016/s0023-6438(95)91398-x
Sreekanth, T.V.M.; Ramanaiah, S.; Lee, K.D.; Reddy, K.S.: Hansen solubility parameters in the analysis of solvent–solvent interactions by inverse gas chromatography. J. Macromol. Sci. Part B Phys. 51, 1256–1266 (2012). https://doi.org/10.1080/00222348.2011.627825
Marcus, Y.: Total and partial solubility parameters of sub- and supercritical ethanol. J. Chem. Thermodyn. 126, 187–189 (2018). https://doi.org/10.1016/j.jct.2018.06.023
Marcus, Y.: Total and partial solubility parameters of supercritical methanol. J. Supercrit. Fluids 111, 43–46 (2016). https://doi.org/10.1016/j.supflu.2016.01.009
Marcus, Y.: Are solubility parameters relevant to supercritical fluids? J. Supercrit. Fluids 38, 7–12 (2006). https://doi.org/10.1016/j.supflu.2005.11.008
Marcus, Y.: Hansen solubility parameters for supercritical water. J. Supercrit. Fluids 62, 60–64 (2012). https://doi.org/10.1016/j.supflu.2011.10.018
Mohammed, S.; Mansoori, G.A.: Molecular insights on the interfacial and transport properties of supercritical CO2/brine/crude oil ternary system. J. Mol. Liq. 263, 268–273 (2018). https://doi.org/10.1016/j.molliq.2018.05.009
Choi, P.; Kavassalis, T.A.; Rudin, A.: Estimation of the three-dimensional solubility parameters of alkyl phenol ethoxylates using molecular dynamics. J. Colloid Interface Sci. 150, 386–393 (1992). https://doi.org/10.1016/0021-9797(92)90208-4
Qing, X.; Kailiang, Y.: Calculation of solubility parameters of organic solvents by molecular dynamics simulation Journal of Jiangsu University. J. Jiangsu Univ. 16, 40–42 (2004)
Linstrom, P.J.; Mallard, W.G. (eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD, 20899; n.d. https://doi.org/10.18434/t4d303.
See Accelrys page: http://accelrys.com/ n.d. Accessed 1 Oct 2018
Sum, H.: COMPASS: an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 102, 7338–7364 (1998)
Acknowledgements
The author acknowledges support from Center for Refining and Petrochemicals at King Fahd University of Petroleum and Minerals (KFUPM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alasiri, H. A Molecular Dynamics Simulation Probe of the Solubility Parameters of Supercritical Water and Methanol. Arab J Sci Eng 44, 9911–9917 (2019). https://doi.org/10.1007/s13369-019-03957-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-019-03957-w