Abstract
Small non-flying mammals represent 44% of Brazilian mammal species and have a wide range of habits and life-history strategies. This wide diversity requires different sampling methods in survey studies. We compared the efficiency of pitfall and live-traps in different vertical positions in relation to the alpha and beta diversity of small mammals in three forest fragments with different levels of conservation and in a continuous area in the southwestern Amazon, Acre state. Captures were carried out using a combination of pitfall traps and live-traps on the ground, understorey, and canopy. Taxonomic identification was performed by morphological and molecular analyses. Alpha diversity was evaluated using Hill numbers (q = 0 and q = 1). The turnover between different types of traps and different vertical strata (beta diversity) was analysed using permutation analysis of variance. Species richness between areas ranged from 6 to 21. The highest species richness was observed in ground traps, and the lowest species richness was observed in the canopy. Live-traps on the ground recorded a greater diversity in two areas. Pitfall traps recorded the greatest number of unique species in three areas. The different types of traps and the different vertical positions acted in a complementary way in the small mammal samplings. The turnover in relation to trap type and stratum indicated the formation of two significantly different groups: ground traps and aboveground traps. However, the use of canopy traps did not contribute significantly to an increase in the estimated species richness and diversity in three of the four localities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Small non-flying mammals, represented by small rodents and didelphid marsupials, represent approximately 40% of Brazilian mammal species (Quintela et al. 2020) and have a wide diversity of habits and life-history strategies. Species vary from 10 to 1500 g in mass; they have terrestrial, fossorial, arboreal, or scansorial habits and show a wide variety of feeding habits — frugivorous, granivorous, folivorous, insectivorous, and omnivorous species (Paglia et al. 2012). Such characteristics give small non-flying mammals a high rate of adaptation and ability to occupy different habitats (Emmons and Feer 1997; Vieira and Monteiro-Filho 2003; Hannibal and Caceres 2010). This wide range of characteristics leads to difficulties in conducting species surveys for this group, resulting in the need for different sampling methods to reduce the large gaps in knowledge related to the occurrence and distribution of small mammals.
Traditionally, small mammals are sampled using live-traps and, in many cases, pitfall traps. Live-traps attract animals with baits and allow the capture of a wide range of species when using different sizes. Pitfalls attract animals by interception and can capture animals that are not attracted by baits or live-traps; thus, they are considered a complementary method to live-traps. Pitfall traps were determinant in estimating the species richness and abundance of rodents and marsupials in forest studies (Quintela et al. 2013; Abreu-Júnior et al. 2016; Bovendorp et al. 2017). However, pitfalls can expose the captured animals to predation and to weather conditions, such as heat and rain (Barros et al. 2015). In addition, they are a more laborious sampling method than live-traps because they require prior installation and permanent maintenance once installed. Another factor to be considered when sampling small mammals is the different use of vertical strata by animals in forest areas due to their great diversity of locomotor habits already mentioned. Thus, sampling in different strata allows a greater range of species to be recorded in a survey. However, sampling small mammals in different strata, especially in the canopy, can be more expensive and laborious.
Despite their ecological importance, small mammals are one of the least studied taxa in the Amazon. This is due to the natural logistical difficulties imposed by the biome, the high degree of vertical stratification of the environment, the high cost involved in small mammal surveys (Gardner et al. 2008), and the ecological characteristics of this taxa, such as the low density of many species and the diversity in the use of space, which require a large and complex sampling effort (Gu and Swihart 2004; Bovendorp et al. 2017; Rodrigues et al. 2020). In addition, land access to several areas in the Amazon is limited, with river transport being of great importance in the region (Peres and Lake 2003; Hernández-Fontes et al. 2021). Thus, geographic and economic difficulties restrict biological studies to few locations in this biome, resulting in many areas with no small mammal species surveys throughout the region (Oliveira et al. 2017). Despite this, both pitfalls and live-traps have already been successfully used in species surveys and biodiversity studies in the Amazon (Santos-Filho et al. 2015; Ardente et al. 2017).
Considering the different vertical strata, there are few studies with small Amazonian mammals that have used canopy traps and verified their efficiency (Patton et al. 2000; Palmeirim et al. 2020). Lambert et al. (2005) evaluated the impact of the use of canopy traps in two areas in the eastern Amazon and found little improvement in the species accumulation curves when canopy samplings were included. Patton et al. (2000), in their classic study of small mammals along the Juruá River, recorded several species on the ground and in traps placed on platforms installed in the canopy but did not sample the understorey, making it difficult to compare strata.
Given the diversity of sampling protocols used in field studies of small mammals and the high costs of field campaigns, understanding the efficiency of different methods (i.e. number of sampling days, type of trap, vertical position) is essential to compare studies and to identify the most adequate trapping arrangement, especially when financial resources are limited (Bovendorp et al. 2017; Rodrigues et al. 2020; Palmeirim et al. 2020). In this sense, our study aimed to compare the efficiency of pitfalls and live-traps and their placement in three different vertical strata (e.g. ground, understorey, and canopy) in relation to small mammal species richness and alpha and beta diversity in four different Amazonian forest sites in the state of Acre, Brazil. Based on those studies, we tested the hypothesis that the different types of traps and their position in the vertical strata act in a complementary way in their efficiency of sampling small mammals; that is, no type of trap or vertical positioning is able to sample all species of small mammals.
Material and methods
Study areas
Our study was conducted in the southwest Brazilian Amazon in the eastern region of the state of Acre. The region is dominated by different phytophysiognomies that cover the Amazon basin, with emphasis on open forests with palm trees, open forests with bamboos characterized by a low density of wood and understorey dominated by the genus Guadua, and few patches of dense forest. The average annual rainfall is 2160 mm, with a monthly variation ranging from 28 to 299 mm (Duarte 2020).
We sampled small mammals in three forest fragments and in a continuous forest area in the Acre River basin, all formed by a mixture of the three vegetation types described above (Fig. 1). The localities sampled were (1) Seringal Cachoeira (SEC), (10°49′S, 68°21′W), in the municipality of Xapuri, an area of continuous primary forest of 24,200 ha; (2) Reserva Florestal Humaitá (RFH) (9°43′S, 67°48′W), in the municipality of Porto Acre, a large forest fragment of approximately 2800 ha of primary and secondary vegetation, surrounded by farms, roads, and by the Acre River in its eastern portion; (3) Fazenda Experimental Catuaba (FEC) (10°04′S, 67°37′W), in the municipality of Senador Guiomard, a forest fragment of 900 ha of primary and secondary forest, surrounded by farms and roads; and (4) Floresta do Parque Zoobotânico (FPZ) (9°57′S, 67°52′W), in the municipality of Rio Branco, an urban forest fragment of approximately 140 ha of forests in different stages of ecological succession.
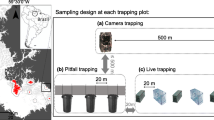
A Location of the four study areas in the eastern part of the state of Acre, Brazil, 1–Seringal Cachoeira (SEC), 2–Reserva Florestal Humaitá (RFH), 3–Fazenda Experimental Catuaba (FEC), 4–Floresta Parque Zoobotânico (FPZ). B Drawing of the platform and scheme of ropes used for canopy sampling. C Scheme of a transect containing the different types of traps and vertical samplings used in the study: Sherman ground, Tomahawk ground, Sherman understorey, Sherman canopy, and Pitfall
Field expeditions took place in March and August 2014 and July and November 2015 in the SEC, RFH, and FPZ. At FEC, expeditions took place in November 2016 and October 2018.
Sampling design
In each of the surveyed areas, we established five to ten linear transects of 225 m spaced from 500 to 1000 m apart. In each transect, 15 capture stations were installed, spaced 15 m apart, where live-traps Sherman® (Sh) or Tomahawk® (Tw) were placed at three different heights of the forest’s vertical stratum (ground, understorey, and canopy). The live-traps were baited with a mixture of bacon, banana, corn, and peanuts. The traps were installed alternately in terms of type and positioning along the 15 capture stations, as follows: (1) a Sherman® (30 × 8 × 9 cm) installed on the ground and a Sherman® (35 × 10 × 12 cm) installed in the canopy; (2) a Tomahawk® (40 × 15 × 15 cm) installed on the ground; and (3) a Sherman® (30 × 8 × 9 cm) installed in the understorey at 1 to 3 m high (Fig. 1). Canopy traps were placed on a platform ~10 m high, hoisted by ropes and pulleys in an adaptation of the technique presented by Lambert et al. (2005) (Fig. 1). At the end of each transect, we installed a pitfall composed of four 60-L buckets spaced at 10 m connected by a guide fence (plastic tarp) 1 m high, arranged in Y.
In each field campaign, the transects were sampled for five consecutive days. All traps were inspected daily, and baits were replaced if necessary. Capture success was calculated as follows: the number of animals captured divided by the trapping effort (number of traps × number of trapping nights) multiplied by 100. The captured animals were transported to a base camp laboratory, where they were properly anaesthetized and euthanized. The animals were captured under the authorization of the Brazilian Government’s Chico Mendes Institute for Biodiversity and Conservation (ICMBio, licence number 13373-1). Captures and animal handling were performed according to the Ethical Committee on Animal Use of the Oswaldo Cruz Foundation (CEUA licence number LW-39/2014) and followed the standard protocols of biosafety of Lemos and D’Andrea (2014). The collected animals were identified through integrated analysis of external and cranial morphology, cytogenetics (karyotype) for rodents, and molecular analysis (cytochrome b sequencing) when necessary (Bonvicino et al. 2005; Gonçalves et al. 2014). Specimens were submitted to taxidermy and their skulls, skins, and tissue were deposited in the mammal collection of the Laboratory of Biology and Parasitology of Wild Reservoir Mammals at Oswaldo Cruz Institute in the state of Rio de Janeiro, Brazil.
Data analysis
The studied areas had differences in their structural characteristics, such as the size of the forest fragment and the type of the surrounding matrix, and in relation to the trapping effort. Because these factors have a great influence on small mammal sampling (Pardini et al. 2005; Borges-Matos et al. 2016), data were analysed and discussed for each locality separately. Moreover, we used individual-based species extrapolation and rarefaction curves as a measure of diversity to compare diversity between traps and strata for each locality. Species-based rarefaction and extrapolation curves based on individuals are more appropriate for comparing communities where there are differences in the sampling effort and in the capture rate amongst them (Gotelli and Colwell 2001). Rarefaction and extrapolation curves were generated to estimate species richness (total number of species) and diversity based on Shannon Hill numbers (Jost 2006). This index is considered the most appropriate for diversity estimates that take into account the distribution of abundance amongst the sampled species (Chao et al. 2014; Hsieh et al. 2016). The Hill number q = 0 considers the number of species recorded. The Hill number q = 1 weights the estimated diversity according to the distribution of species abundance, thus representing the number of equally common species within a community that are required to provide a specific Shannon diversity index value (Chao et al. 2014).
Rarefaction and extrapolation curves were generated using 100 bootstrap iterations to obtain an 84% confidence interval (CI; i.e. 1.41 times SE). We consider 84% CIs more appropriate for comparing curves, as 95% CIs may be conservative for comparison between groups (Payton et al. 2003; Camargo et al. 2018). Furthermore, in simulations, a CI of 84% came closest to a significance level of 0.05 (MacGregor-Fors and Payton 2013). However, we considered that our sampled communities have high diversity, registering the presence of rare species in the sample, which produces high confidence intervals, making it difficult to compare amongst groups (Chao and Jost 2012). For this reason, extrapolations of species diversity indices estimated in each curve were limited to twice the abundance of the treatment under analysis (Colwell et al. 2012).
To evaluate species diversity between methods, we estimated rarefaction and species extrapolation curves for pitfalls and live-trap captures and for each strata based on individuals sampling for each area. Species rarefaction and extrapolation curves were generated in the R Core Team 4.0.5 software in the iNEXT package (Hsieh et al. 2016).
Posteriorly, the species turnover between the different types of traps and different vertical strata (beta diversity) was analysed using permutation analysis of variance (PERMANOVA) to test the hypothesis that the different types of traps act in a complementary way for small mammal sampling as follows. First, we calculated the Jaccard distance, which considers only the species composition, and the Bray-Curtis distance, which considers the abundance of each species, using Hellinger transformation to reduce the effect of very abundant species (Borcard et al. 2011). Then, principal coordinate analyses (PCoAs) and pairwise comparisons between the different types of traps and vertical strata of the Jaccard and Bray-Curtis distances were performed using PERMANOVA. This analysis allows the comparison of community dissimilarities between one or more groups by permutations. Analyses were performed with 10,000 iterations, with the sampling site as a replica. These analyses were performed using the vegan package (Oksanen et al. 2020) in R Core Team 4.0.5 software.
Results
The SEC area had a total of 17 species, five of which were captured exclusively in pitfalls, one exclusively in ground live-traps, and four exclusively above ground (Table 1; Fig. 2). The capture success was higher in pitfalls (6.25) than in ground live-traps (4.7). Caluromys lanatus was captured exclusively in the canopy. Proechimys gardneri had high abundance in live-traps on the ground. In this area, the pitfalls showed greater species richness and diversity than the live-traps on the ground without overlaps in the confidence intervals (Fig. 3). However, in relation to the vertical strata, this difference was not observed, with a great overlap in the rarefaction and extrapolation curves between the strata (Fig. 4).
Number of exclusive species for each sampling technique and each stratum at four sampling sites in the Acre River basin, state of Acre, Brazil. FPZ, Floresta do Parque Zoobotânico, FEC, Fazenda Experimental Catuaba, RFH, Reserva Florestal Humaitá, SEC, Seringal Cachoeira, SH, Sherman, TW, Tomahawk, LT, live-traps (Sherman + Tomahawk)
Individual-based rarefaction and extrapolation species accumulation curves of small mammals with 84% confidence intervals using the Hill numbers for pitfall and live-traps on the ground at four sampling sites in the river Acre basin, state of Acre, Brazil. Sh, Sherman, Tw, Tomahawk. SEC, Seringal Cachoeira, RFH, Reserva Florestal Humaitá, FEC, Fazenda Experimental Catuaba, FPZ, Floresta do Parque Zoobotânico
Individual-based rarefaction and extrapolation species accumulation curves of small mammals with 84% confidence intervals using the Hill numbers for ground, understorey, and canopy traps at four sampling sites in the Acre River basin, state of Acre, Brazil. SEC, Seringal Cachoeira, RFH, Reserva Florestal Humaitá, FEC, Fazenda Experimental Catuaba, FPZ, Floresta do Parque Zoobotânico
In RFH, 21 species were recorded, four of which were captured exclusively in pitfalls, four exclusively in live-traps on the ground, and two exclusively above ground (Table 1; Fig. 2). No species were captured exclusively in the canopy (Fig. 2). Marmosa constantiae and Marmosa rutteri stood out for their high abundance in canopy traps, although they were also captured in the other two strata. The capture success was 3.9 in pitfalls, 5.3 in ground live-traps, and 4.3 above ground, with the highest success in Sherman ground. The rarefaction and extrapolation curves indicated similar species richness and diversity between pitfalls and live-traps on the ground for both q = 0 and q = 1 (Fig. 3). However, comparing the strata, we observed greater species richness and diversity on the ground in relation to the other two strata. In addition, the understorey had higher indices than the canopy without overlaps in the confidence intervals for q = 1 (Fig. 4).
Fifteen species were captured in the FEC, of which three were captured exclusively in pitfalls, two exclusively in live-traps on the ground, and seven exclusively above ground, amongst which two were captured exclusively in the canopy, which were Mesomys hispidus and Rhipidomys leucodactylus (Table 1; Fig. 2). The capture success was higher in pitfalls (4.5) than in live-traps on the ground (2.1). The capture success above ground was 2.5, being 3.2 in the canopy. Rarefaction and extrapolation curves indicated greater species richness and diversity in pitfall traps than in live-traps on the ground, despite the overlapping confidence intervals in the q = 0 curve (Fig. 3). Regarding the strata, there was a great overlap in the rarefaction and extrapolation curves, both in q = 0 and in q = 1 (Fig. 4). In this area, Monodelphis glirina showed high abundance in pitfalls, Proechimys gardneri showed high abundance in live-traps on the ground, and Marmosa constantiae and Marmosa rutteri had high abundance above ground (Table 1).
In the FPZ area, the total species richness was six. This result was similar between pitfall and Sherman ground traps and had a small variation amongst strata (Table 1). The highest richness was detected in Tomahawk ground. Considering ground traps only, live-traps recorded four species whereas pitfalls recorded two (Table 1). The capture success was also higher in live-traps on the ground (1.2) than in pitfalls (0.75). (Table 1). In this area, Marmosa constantiae was captured exclusively above ground, Monodelphis peruviana was captured exclusively in pitfalls, three species were captured exclusively in live-traps on the ground, and none was captured exclusively in canopy traps (Table 1; Fig. 2). Rarefaction and extrapolation curves showed a large overlap in the confidence intervals between pitfalls and ground live-traps and amongst strata, either for q = 0 or for q = 1 (Figs. 3 and 4), indicating small differences between techniques and between strata. This small difference can be attributed to a greater abundance of Philander canus in live-traps on the ground, also captured in pitfalls and in the understorey.
In the analysis of turnover in relation to the types of traps and strata, the PERMANOVA indicated the formation of two significantly different groups: (a) ground traps (Pitfall, Sherman ground and Tomahawk ground) and (b) aboveground traps (understorey and canopy traps) (Fig. 5). The pairwise comparisons showed significant differences between groups for both Jaccard (F = 1.672, R2 = 0.31, p = 0.021) and Bray-Curtis methods (F = 2.275, R2 = 0.38, p = 0.007), except for the comparison between Sherman ground and canopy traps (Table 2).
Spatial variation in small mammal beta diversity at four sampling sites in the Acre River basin, state of Acre, Brazil, amongst different trap types (Pitfall, Sherman ground, and Tomahawk ground) and different vertical strata (ground, understorey, and canopy) based on principal coordinate analysis (PCoA) using Jaccard (composition only) and Bray-Curtis (species composition and abundance) distances, respectively, tested by permutational multivariate analysis of variance (PERMANOVA; p < 0.05). Letters a and b indicate the categories significantly different in pairwise comparisons. Sh, Sherman, Tw, Tomahawk. SEC, Seringal Cachoeira, RFH, Reserva Florestal Humaitá, FEC, Fazenda Experimental Catuaba, FPZ, Floresta do Parque Zoobotânico
Discussion
As expected, live-traps and pitfall traps acted in a complementary way in relation to species composition, supporting our hypothesis. The beta diversity observed between trap types and between the vertical strata of the forest (Fig. 5) showed important differences in species composition in this study. Considering the traps on the ground, pitfalls registered a higher number of overall species and of exclusive species in SEC and FEC, the largest areas. For both species richness and alpha diversity, the greatest difference found between the captures in pitfalls and in live-traps on the ground was in the SEC locality, which is the continuous area, where five species were captured only in pitfalls. In the FPZ, which is an urban fragment and the smallest area sampled, differences in species richness and diversity between trap types were not observed, which also occurred for the RFH locality, although the number of exclusive species found in ground live-traps was higher than that in pitfalls in the FPZ.
In the two localities where differences between pitfall and live-traps (SEC and FEC) were found, this difference increased considering the diversity index q = 1 in relation to the species richness (q = 0), with greater diversity using pitfalls. Diversity takes into account not only species richness but also species composition and relative abundance. Trapping methods differ in their capture efficiencies according to species locomotor habit or use of space. Therefore, the species captured differed amongst trap types or strata, with species exclusive to each method. Vertical stratification is a widespread phenomenon in vertebrate community structure (Basham et al. 2022). Most of the species found are terrestrial or scansorial (Table 1), following the pattern of small Amazonian mammals (Ardente et al. 2017; Palmeirim et al. 2020). This information helps to understand how individuals are distributed and adapt to different environments, because habitat disturbances can change the vertical use behaviour in species with greater plasticity in use of space (Delciellos et al. 2017). Additionally, there were large differences in the abundances of certain species that were captured in more than one method, which is reflected in the diversity indices and was clearer in the most preserved areas. Furthermore, the placement of live-traps in the upper strata was also a considerable factor. For example, some species of the genera Marmosa and Proechimys were very abundant in live-traps with few captures in pitfalls, whilst for Monodelphis and Neacomys, the opposite occurred, although they were captured in both methods.
Studies that evaluated the efficiency of pitfall traps mostly recommended the use of the technique (Umetsu et al. 2006; Santos-Filho et al. 2015; Bovendorp et al. 2017; Ardente et al. 2017; Rodrigues et al. 2020). These traps are highly recommended for the Amazon, as in our results, pitfalls capture species that are not captured in live-traps (Santos-Filho et al. 2015; Ardente et al. 2017).
Another outstanding aspect of our captures with pitfall traps is the high number of small species captured with this method, especially of the genera Monodelphis and Neacomys (Table 1). In our study, the average body mass of the individuals captured in pitfalls was 33.9 g (n = 65), whilst in Shermans on the ground, it was 104.4 g (n = 84), and in Tomahawks on the ground, it was 176.7 g (n = 71). This morphological difference amongst the captured species can influence studies of the functional ecology of small mammals because body mass is often used as a trait related to species metabolism (Lovegrove 2005; Bovendorp et al. 2019; Palmeirim et al. 2021). It is also important to highlight that when using pitfalls, the animals are more exposed to predation and aggressive interactions than when using live-traps. Thus, daily inspection, the use of polystyrene platforms inside the buckets to avoid animal drowning in case of rain, and the removal of accumulated water from inside the buckets are essential to avoid accidental deaths (Barros et al. 2015).
Regarding the vertical strata, only the RFH locality showed differences in captures amongst strata, with a small difference between understorey and canopy captures. These differences were clearer when considering the diversity index compared with the richness index. This is because there are large differences in the abundance of small mammals captured between the understorey and the canopy strata, which is reflected more in species diversity than in species richness. In addition, when analysing the beta diversity between strata, the PERMANOVA results showed differences between the ground traps and the upper traps, without a significant difference between the understorey and canopy. Thus, the use of canopy traps did not contribute to an increase in the estimated species richness and diversity in three of the four localities studied, although FEC and SEC showed, respectively, two and one exclusive species in the canopy. Considering these three species amongst the 27 recorded species in the four localities, only Caluromys lanatus was exclusively captured in the canopy. This marsupial has an average body ass of 412 g and generally occupies the upper strata of the forest (Faria et al. 2019), although this species and its congener C. philander have already been captured in live-traps in the understorey and even on the ground in different areas in the Amazon (Ardente 2012; Santos-Filho et al. 2015; Borges-Matos et al. 2016). Lambert et al. (2005), in a study in the eastern Amazon, also found that traps in the canopy did not contribute to the diversity of small mammal species sampled in relation to the other strata. These authors argued that the canopy of the studied sites in the state of Pará was not high (10.2 m), considering Amazon standards of canopy heights, which vary from 26 to 30 m (Alencar 2020). The average canopy height of our study was similar to that of Lambert et al. (11.2 m), which may have reduced the segregation between the two upper strata.
Five species of arboreal mammals, which were not captured in this study, were expected to occur: the echimid rodents Dactylomys dactylinus, Isothrix bistriata, and Makalata macrura and the marsupials Caluromysiops irrupta and Glironia venusta (Patton et al. 2015; Faria et al. 2019). The genera Caluromysiops and Glironia are hardly captured in live-traps in the Amazon (Ardente et al. 2013; Barbosa et al. 2016). There is only one record of Glironia venusta in the canopy in the Carajás National Forest, state of Pará (Ardente 2012). Amongst rodents, D. dactylinus has never been captured in traps. This species is well known for its characteristic nocturnal vocalization, mainly in forest areas with bamboo (Patton et al. 2000, 2015). The two other rodent species, I. bistriata and M. macrura, also have few capture records in live-traps and in pitfall traps (Ardente et al. 2017; Palmeirim et al. 2019). An important factor to consider is the possibility of a strong trap shyness in these arboreal species. Palmeirim et al. (2020) associated captures of the genera Echimys and Isothryx and even of the sciurid Guerlinguetus with the increase in the number of days of the sampling campaign. According to these authors, performing samplings for more than 8 days can facilitate the capture of these genera because the aversion of the animals to the installed traps may decrease over time. Thus, the absence of these arboreal mammals in our study areas and in other surveys in the Amazon seems to be more related to intrinsic characteristics of the species, such as low abundance or trap-shyness than to the use of traps in the canopy.
Despite the small contribution of canopy traps to the present study, individuals of the genus Marmosa were more collected in the canopy (55) than in the understorey (23). Marmosa is a very representative genus and one of the most abundant genera in Amazon surveys (Borges-Matos et al. 2016; Ardente et al. 2017; Palmeirim et al. 2020). This genus is classified as arboreal (Emmons and Feer 1997; Paglia et al. 2012), although it is more registered in the understorey than in the canopy (Lambert et al. 2005) and has many captures registered on the ground (Santos-Filho et al. 2015). Therefore, despite the high capture rate in canopy traps, the use of live-traps in understorey is effective for the detection of genus Marmosa in the Amazon forest. An alternative option could be to use traps of different sizes in the understorey, such as Sherman and Tomahawk, without the need for the laborious installation of canopy traps.
We recommend the authors who carry out non-flying small mammal surveys to separate the results of the captures amongst strata to provide a deep discussion concerning the use of the vertical space in complex environments such as the Amazon. Some of the main studies conducted in this biome did not show their results by strata, even though they sampled at different heights of the forest (Ardente et al. 2016; Borges-Matos et al. 2016; Palmeirim et al. 2020). Even in the Atlantic forest, which is much better surveyed than the Amazon, there is a lack of this kind of information for small mammals (Bovendorp et al. 2017). Such information may facilitate the investigation of how local characteristics influence the use of vertical space by different species. In our study sites, there was a predominance of open bamboo forests, which are characterized by an understorey obstructed by the dominance of bamboo species (Guadua sp.) (Silveira 1999; Griscom et al. 2007). This dense understorey can favour the capture of arboreal animals (Basham et al. 2022), as a more obstructed understorey can offer more resources for individuals to move around. Thus, the traps placed in the understorey in such forests probably supply live-traps installed in the canopy.
Live-traps and pitfalls proved to be complementary, and no method was more efficient than the others in all localities. The species composition and species diversity observed in this study were more affected by trap type than the species richness observed, which was quite similar between the two methods, showing higher estimates for pitfalls in two amongst the four localities. The use of traps in the upper strata (canopy and understorey) also proved to be a complementary method because it allows the capture of species that are not very abundant on the ground or that are exclusively arboreal. Significant differences between strata were observed only in one of the localities (RFH), where we registered a lower diversity in the canopy than in the understorey, which, in turn, we registered a lower diversity than on the ground. The diversity and species composition registered in our study were also more affected than richness when comparing the strata, mainly between the ground and the upper strata, without a significant difference between the understorey and the canopy. Therefore, we recommend researchers to use live-traps on the ground and in the understorey in small mammal surveys in the Amazon region, because canopy traps have shown a lower cost-benefit ratio when compared to understorey traps. Furthermore, the use of pitfalls should always be considered as some species are not captured in live-traps.
References
Abreu-Júnior EF, Freitas MA, Lapenta MJ, Venâncio NM, França DPF, Percequillo AR (2016) Marsupials and rodents (Didelphimorphia and Rodentia) of upper Rio Acre, with new data on Oxymycterus inca Thomas, 1900 from Brazil. Check List 12:1956. https://doi.org/10.15560/12.5.1956
Alencar GM (2020) Relações entre a ocorrência de raízes acima do solo e fatores individuais e ambientais na Reserva Florestal Adolpho Ducke. Dissertation. Instituto Nacional de Pesquisas da Amazônia - INPA
Ardente N, Gettinger D, Fonseca R, Bergallo HG, Martins-Hatano F (2013) Mammalia, Didelphimorphia, Didelphidae, Glironia venusta Thomas, 1912 and Chironectes minimus (Zimmermann, 1780): distribution extension for eastern Amazonia. Check List 9:1104. https://doi.org/10.15560/9.5.1104
Ardente NC (2012) A comunidade de pequenos mamíferos em áreas de savana metalófila e floresta ombrófila densa na Floresta Nacional de Carajás, PA: estrutura, estratificação e impacto da mineração. Dissertation. Universidade do Estado do Rio de Janeiro
Ardente NC, Ferreguetti ÁC, Gettinger D, Leal P, Martins-Hatano F, Bergallo HG (2017) Differencial efficiency of two sampling methods in capturing non-volant small mammals in an area in eastern Amazonia. Acta Amazon 47:123–132. https://doi.org/10.1590/1809-4392201602132
Ardente NC, Ferreguetti ÁC, Gettinger D, Leal P, Mendes-Oliveira AC, Martins-Hatano F, Bergallo HG (2016) Diversity and impacts of mining on the non-volant small mammal communities of two vegetation types in the Brazilian Amazon. PLoS One 11:e0167266. https://doi.org/10.1371/journal.pone.0167266
Barbosa JL, Custódio RJ, Brandão MV (2016) Rediscovery and range extension of the black-shouldered opossum Caluromysiops irrupta Sanborn, 1951 (Didelphimorphia, Didelphidae) in Brazil. Mammalia 80:325–328. https://doi.org/10.1515/mammalia-2014-0147
Barros CS, Püttker T, Pinotti BT, Pardini R (2015) Determinants of capture-recapture success: an evaluation of trapping methods to estimate population and community parameters for Atlantic forest small mammals. Zoologia (Curitiba) 32:334–344. https://doi.org/10.1590/S1984-46702015000500002
Basham EW, Baecher JA, Klinges DH, Scheffers BR (2022) Vertical stratification patterns of tropical forest vertebrates: a meta-analysis. Biological Revi. https://doi.org/10.1111/brv.12896
Bonvicino CR, Otazú IB, Vilela JF (2005) Karyologic and molecular analysis of Proechimys Allen, 1899 (Rodentia, Echimyidae) from the Amazonian region. Arquivos do Museu Nacional, Rio de Janeiro 63:191–200
Borcard D, Gillet F, Legendre P (2011) Numerical Ecology with R. Springer, New York. https://doi.org/10.1007/978-1-4419-7976-6
Borges-Matos C, Aragón S, Silva MNF, Fortin MJ, Magnusson WE (2016) Importance of the matrix in determining small-mammal assemblages in an Amazonian forest-savanna mosaic. Biol Conserv 204:417–425. https://doi.org/10.1016/j.biocon.2016.10.037
Bovendorp RS, Brum FT, McCleery RA, Baiser B, Loyola R, Cianciaruso MV, Galetti M (2019) Defaunation and fragmentation erode small mammal diversity dimensions in tropical forests. Ecography 42:23–35. https://doi.org/10.1111/ecog.03504
Bovendorp RS, McCleery RA, Galetti M (2017) Optimising sampling methods for small mammal communities in Neotropical rainforests. Mamm Rev 47:148–158. https://doi.org/10.1111/mam.12088
Camargo NF, Sano NY, Vieira EM (2018) Forest vertical complexity affects alpha and beta diversity of small mammals. J Mammal 99:1444–1454. https://doi.org/10.1093/jmammal/gyy136
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. https://doi.org/10.1890/13-0133.1
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547. https://doi.org/10.1890/11-1952.1
Colwell RK, Chao A, Gotelli NJ, Lin SY, Mao CX, Chazdon RL, Longino JT (2012) Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. J Plant Ecol 5:3–21. https://doi.org/10.1093/jpe/rtr044
Delciellos AC, Ribeiro SE, Vieira MV (2017) Habitat fragmentation effects on fine-scale movements and space use of an opossum in the Atlantic forest. J Mammal 98:1129–1136. https://doi.org/10.1093/jmammal/gyx043
Duarte AF (2020) A inserção da Fazenda Catuaba no clima do Acre. In: Silveira M, Guilherme E, Vieira LJS (eds) Fazenda Experimental Catuaba: O seringal que virou laboratório-vivo em uma paisagem fragmentada no Acre. Stricto Sensu Editora, Rio Branco, pp 91–119
Emmons LH, Feer F (1997) Neotropical rainforest mammal: a field a guide. University of Chicago Press, Chicago
Faria MB, Lanes RO, Bonvicino CR (2019) Marsupiais do Brasil. Amélie Editorial, São Caetano do Sul
Gardner TA, Barlow J, Araujo IS et al (2008) The cost-effectiveness of biodiversity surveys in tropical forests. Ecol Lett 11:139–150. https://doi.org/10.1111/j.1461-0248.2007.01133.x
Gonçalves RP, Oliveira JA (2014) An integrative appraisal of the diversification in the Atlantic forest genus Delomys (Rodentia: Cricetidae: Sigmodontinae) with the description of a new species. Zootaxa. https://doi.org/10.11646/zootaxa.3760.1.1
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. https://doi.org/10.1046/j.1461-0248.2001.00230.x
Griscom BW, Daly DC, Ashton MS (2007) Floristics of bamboo-dominated stands in lowland Terra-Firma Forests of Southwestern Amazonia. J Torrey Bot Soc 134:108–125
Gu W, Swihart RK (2004) Absent or undetected? Effects of non-detection of species occurrence on wildlife-habitat models. Biol Conserv 116:195–203. https://doi.org/10.1016/S0006-3207(03)00190-3
Hannibal W, Caceres NC (2010) Use of vertical space by small mammals in gallery forest and woodland savannah in south-western Brazil. Mammalia 74:247–255. https://doi.org/10.1515/MAMM.2010.007
Hernández-Fontes JV, Maia HWS, Chávez V, Silva R (2021) Toward more sustainable river transportation in remote regions of the Amazon, Brazil. Appl Sci 11:2077. https://doi.org/10.3390/app11052077
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. https://doi.org/10.1111/2041-210X.12613
Jost L (2006) Entropy and diversity. Oikos 113:363–375. https://doi.org/10.1111/j.2006.0030-1299.14714.x
Lambert TD, Malcolm JR, Zimmerman BL (2005) Variation in small mammal species richness by trap height and trap type in southeastern Amazonia. J Mammal 86:982–990
Lemos ERS, D’Andrea PS (2014) Trabalho de campo com animais: Procedimentos, riscos e biossegurança. FIOCRUZ, Rio de Janeiro
Lovegrove BG (2005) Seasonal thermoregulatory responses in mammals. J Comp Physiol B 175:231–247. https://doi.org/10.1007/s00360-005-0477-1
MacGregor-Fors I, Payton ME (2013) Contrasting diversity values: statistical inferences based on overlapping confidence intervals. PLoS One 8:e56794. https://doi.org/10.1371/journal.pone.0056794
Oksanen J, Blanchet FG, Friendly M et al (2020) vegan: community ecology package. R Core Team https://cran.r-project.org/web/packages/vegan/index.html. Accessed 12 Jul 2021
Oliveira U, Soares-Filho BS, Paglia AP et al (2017) Biodiversity conservation gaps in the Brazilian protected areas. Sci Rep 7:9141. https://doi.org/10.1038/s41598-017-08707-2
Paglia AP, Rylands AB, Herrmann G et al (2012) Lista Anotada dos Mamíferos do Brasil, 2a. Conservation International, Arlington
Palmeirim AF, Benchimol M, Peres CA, Vieira MV (2019) Moving forward on the sampling efficiency of neotropical small mammals: insights from pitfall and camera trapping over traditional live trapping. Mamm Res 64:445–454. https://doi.org/10.1007/s13364-019-00429-2
Palmeirim AF, Farneda FZ, Vieira MV, Peres CA (2021) Forest area predicts all dimensions of small mammal and lizard diversity in Amazonian insular forest fragments. Landsc Ecol 36:3401–3418. https://doi.org/10.1007/s10980-021-01311-w
Palmeirim AF, Peres CA, Vieira MV (2020) Optimizing small mammal surveys in Neotropical fragmented landscapes while accounting for potential sampling bias. Mamm Biol 100:81–90. https://doi.org/10.1007/s42991-020-00012-2
Pardini R, Souza SM, Braga-Neto R, Metzger JP (2005) The role of forest structure, fragment size and corridors in maintaining small mammal abundance and diversity in an Atlantic forest landscape. Biol Conserv. https://doi.org/10.1016/j.biocon.2005.01.033
Patton JL, Pardiñas UFJ, D’Elía G (2015) Mammals of South America, vol 2. University of Chicago Press, Chicago
Patton JL, Silva MNF, Malcolm JR (2000) Mammals of the Rio Juruá and the evolutionary and ecological diversification of Amazonia. Bull Am Mus Nat Hist 244:1. https://doi.org/10.1206/0003-0090(2000)244%3C0001:MOTRJA%3E2.0.CO;2
Payton ME, Greenstone MH, Schenker N (2003) Overlapping confidence intervals or standard error intervals: what do they mean in terms of statistical significance? J Insect Sci. https://doi.org/10.1093/jis/3.1.34
Peres CA, Lake IR (2003) Extent of nontimber resource extraction in tropical forests: accessibility to game vertebrates by hunters in the Amazon Basin. Conserv Biol 17:521–535. https://doi.org/10.1046/j.1523-1739.2003.01413.x
Quintela FM, Gonçalves BI, Trindade GE, Santos MB, Tozetti AM (2013) Pequenos mamíferos não-voadores (Didelphimorphia, Rodentia) em campos litorâneos do extremo sul do Brasil. Biota Neotropica 13:284–289
Quintela FM, Rosa CA, Feijó A (2020) Updated and annotated checklist of recent mammals from Brazil. An Acad Bras Cienc 92:1–57. https://doi.org/10.1590/0001-3765202020191004
Rodrigues DP, Skupien FL, Oliveira SJ, Lima DO (2020) Small mammals in fragments of Atlantic forest: species richness answering to field methods and environment. J Trop Ecol 36:101–108. https://doi.org/10.1017/S0266467420000048
Santos-Filho M, Lázari PR, Sousa CPF, Canale GR (2015) Trap efficiency evaluation for small mammals in the southern Amazon. Acta Amazon 45:187–194. https://doi.org/10.1590/1809-4392201401953
Silveira M (1999) Ecological aspects of bamboo-dominated forest in southwestern Amazonia: an ethnoscience perspective. Ecotropica 5:213–216
Umetsu F, Naxara L, Pardini R (2006) Evaluating the efficiency of pitfall traps for sampling small mammals in the neotropics. J Mammal 87:757–765. https://doi.org/10.1644/05-MAMM-A-285R2.1
Vieira EM, Monteiro-Filho ELA (2003) Vertical stratification of small mammals in the Atlantic rain forest of south-eastern Brazil. J Trop Ecol 19:501–507. https://doi.org/10.1017/S0266467403003559
Acknowledgements
We are grateful to the field team of Msc. André L M. Botelho (IFAC), team of Dra. Luciana Medeiros (UFAC), and LABPMR/FIOCRUZ, especially Michele Santos and Bernardo Teixeira. We are grateful to Dra. Maíra Benchimol for the suggestions on a previous version of the manuscript and to Izailene Saar and Queren Hapuque for the help in Fig. 1.
Funding
This work was supported by grants of Conselho Nacional de Pesquisa (CNPq), Fundação de Amparo à Pesquisa do Acre (FAPAC), and Programa de Pesquisa em Biodiversidade (PPBio) via CNPq 428213/2016-2, CNPq 157533/2015-8, CNPq 439208/2018-1, PPBio/CNPq 457540/2012-5, and PPSUS/FAPAC 001/2015.
Author information
Authors and Affiliations
Contributions
Conceptualization: André L. M. Botelho, Paulo S. D’Andrea, Rosana Gentile, Pedro Zanata, Marcos Silveira; methodology: André L. M. Botelho, Paulo S. D’Andrea, Charle F. Crisóstomo; investigation: André L. M. Botelho, Paulo S. D’Andrea, Charle F. Crisóstomo, Pedro Zanata, Camila Lúcio, Cibele Bonvicino; formal analysis: André L. M. Botelho, Paulo S. D’Andrea, Rosana Gentile; writing: André L. M. Botelho, Paulo S. D’Andrea, Rosana Gentile; funding acquisition: Paulo S. D’Andrea, Charle F. Crisóstomo, Marcos Silveira; resources: Paulo S. D’Andrea, Cibele Bonvicino.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Thales Renato Ochotorena de Freitas
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Botelho, A.L.M., D’Andrea, P.S., Crisóstomo, C.F. et al. Evaluating the efficiency of different sampling techniques to survey non-flying small mammals in the Amazon. Mamm Res 69, 9–22 (2024). https://doi.org/10.1007/s13364-023-00711-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-023-00711-4