Abstract
The spotted-tailed quoll (Dasyurus maculatus) is an endangered mesopredator endemic to Australia. It is generally considered a forest-dependent species associated with large, intact forested habitats. In Australia’s mainland, quoll research has typically been conducted in contiguous forest, and consequently, the species’ presumed forest-dependency might reflect sampling bias rather than preferred habitat niche. Recent studies have revealed that quolls also persist in fragmented agricultural landscapes, raising questions about their true habitat requirements and preferences. In this study, we investigated quoll habitat use within a fragmented agricultural landscape in mainland Australia. We deployed 42 lured camera traps to determine quoll habitat preferences across four broad vegetation types (open grassland, grassy woodland, dry sclerophyll forest, and wet sclerophyll forest) based on quoll activity and occupancy. Quolls were detected in all vegetation types, and quoll activity indicated a preference for dry sclerophyll forest and grassy woodlands, although this preference varied depending on the time of year. Our results suggest that quoll habitat use in mainland Australia is more flexible than previously assumed, and we recommend further research on factors that may influence habitat preference such as prey availability and seasonal behavior. Understanding the factors that drive habitat use by quolls outside of contiguous forested landscapes will inform and improve conservation and management strategies to ensure critical habitat for the species is protected and retained in an increasingly fragmented landscape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat loss is one of the main causes of biodiversity loss around the world as humans continue to modify and destroy natural habitats (Rands et al. 2010). Approximately a third of the world’s forests have been cleared, with around 5 million ha cleared annually (Ritchie and Roser 2021). Habitat loss generally causes fragmentation, whereby habitat becomes divided into smaller and more isolated fragments surrounded by a matrix of human-modified land. Species within these habitat fragments are typically vulnerable to isolation and exposed to anthropogenic impacts (Lindenmayer and Fischer 2013).
Mammalian predators are particularly susceptible to habitat fragmentation as they often require relatively large home ranges, occur at naturally low population densities, and have specialized niche requirements (Crooks et al. 2011; Gittleman et al. 2001). Globally, native predator populations are declining as a result of the associated impacts of habitat loss and fragmentation (Chapron et al. 2014; Crooks et al. 2011; Crooks 2002) and subsequent increased conflict with other predators and humans (Farris et al. 2015; Remonti et al. 2012; Ripple et al. 2014). For example, habitat fragmentation has altered habitat use by long-tailed weasels (Mustela frenata) in America (Gehring and Swihart 2003, 2004) as well as the güiña (Leopardus guigna) in Chile (Schüttler et al. 2017). As landscapes continue to be modified and degraded, survival of predators will depend on their ability to persist in human-modified landscapes (Kremen and Merenlender 2018). Some native predator species are able to persist within or even benefit from modified habitats, particularly where resource availability is increased or supplemented (Bateman and Fleming 2012; Crooks 2002; Swihart et al. 2003). For example, pine martens (Martes martes) can exist in agricultural landscapes in Europe (Mergey et al. 2012; Pereboom et al. 2008; Weber et al. 2018) utilizing supplemental prey and den resources (Caryl et al. 2012). Similarly, raccoons (Procyon lotor) in North America are well adapted to human-modified landscapes, utilizing croplands and urban habitats for foraging and denning (Beasley et al. 2007b; Henner et al. 2004).
The spotted-tailed quoll (Dasyurus maculatus, hereafter referred to as “quoll”) is a medium-sized (1.2–4.2 kg, Belcher 2003) marsupial carnivore which occurs throughout eastern Australia, including the island state of Tasmania (Jones et al. 2001). It is known to occur in a range of habitats including wet and dry sclerophyll forests, woodlands, and rainforests (Belcher and Darrant 2004; Claridge et al. 2005; Glen and Dickman 2006b). Quolls are generally considered a forest-dependent species (Belcher 2004), typically associated with structurally complex contiguous forests that offer an abundance of hollow-bearing trees, logs, and burrows, which provide suitable den sites and support high densities of prey (Belcher and Darrant 2006; Claridge et al. 2005; Glen et al. 2011).
Currently, quolls are listed as “near threatened” on the IUCN Red List of Threatened Species, and Australia’s mainland population is listed as endangered under the Commonwealth Environment Protection and Biodiversity Conservation Act 1999. Since European colonization in the late eighteenth century, quolls have suffered a 50–90% decline in their range (Maxwell et al. 1996), with habitat loss being considered the greatest threat to quoll survival (Long and Nelson 2010). Additionally, introduced predators such as feral cats (Felis catus) and red foxes (Vulpes vulpes) threaten quolls through resource competition and predation (Jones et al. 2014). Foxes, in particular, are known to exhibit strong dietary overlap with quolls (Glen and Dickman 2008; Glen et al. 2011), and there is some evidence of interspecific killing of quolls by foxes (Körtner et al. 2004). In southeastern mainland Australia, potential competitive interactions between sympatric quolls and foxes (Henderson et al., 2021) may exacerbate the impacts of habitat loss on quoll populations in fragmented landscapes, potentially restricting quolls to more protected intact forested habitats.
Recent studies in Tasmania (Andersen et al. 2017; Hamer et al. 2021; Jones et al. 2021), as well as in New South Wales (Henderson et al. 2022; Henderson et al. 2021), indicate that quolls are able to persist in fragmented landscapes. However, information on how quolls use these fragmented landscapes is limited to research in Tasmania. Troy (2014) suggested that habitat preferences of quolls in Tasmania are more flexible than in mainland Australia likely due to the absence of interspecific competition with foxes. In mainland Australia, quoll research has predominately been conducted in contiguous forested landscapes. Consequently, their dependence on intact forests is only assumed and could instead reflect sampling bias. Henderson et al. (2021) recently found that quolls and foxes coexisted spatially and temporally within a fragmented landscape in mainland Australia, which was possibly facilitated by low fox density. Therefore, mainland quolls living in some fragmented landscapes could demonstrate similar habitat flexibility to quolls in Tasmania, although this is currently unknown.
In our study, we investigated the habitat use by quolls in mainland Australia in a landscape comprising forested habitat fragments interspersed with open grassland habitat. By identifying essential habitat features to be retained and protected at the landscape level, information on how quolls use fragmented landscapes can help inform relevant conservation and management strategies. We specifically sought to determine whether quoll habitat use within a fragmented landscape was similar across four broad vegetation types (open grassland, grassy woodland, dry sclerophyll forest, and wet sclerophyll forest) or whether quolls demonstrated a preference for forested vegetation types and avoided open grassland. We hypothesized that quolls would prefer dry and wet sclerophyll forests, as mainland quoll occurrence is typically associated with these forest types (Belcher 2004; Catling et al. 2002; McLean et al. 2015) and avoid open grasslands due to the increased risk of interspecific competition and exposure to predators.
Materials and methods
Study site and design
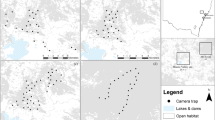
The study was conducted in a biodiversity offset area and adjacent private land (approximately 30 km2) located in the Hunter Valley region of New South Wales, Australia (Fig. 1). The study site was defined by private property and biodiversity site boundaries, and comprised a mix of sclerophyll forests and grassy eucalypt woodlands fragmented by mining and agriculture, and interspersed with open grassland paddocks (Fig. 1). The study site was divided into four broad vegetation types (open grassland, grassy woodland, dry sclerophyll forest, and wet sclerophyll forest; Table 1). The proportion of each vegetation type available within the study site was calculated using ArcGIS version 10.4.1 (ESRI 2015). The study site was then divided into 2 km2 hexagonal cells (with some cells excluded due to access constraints), resulting in 42 accessible cells. Each accessible cell was systematically assigned a vegetation type based on the dominant vegetation within that cell while also ensuring that the proportion of cells per vegetation type was similar to the proportion of available vegetation within the study site (Table 1).
Camera trapping
To assess quoll habitat preferences, we deployed Reconyx HC600 camera traps (Reconyx, Holmen, USA) across all accessible sampling units (n = 42, Fig. 1), and camera placement targeted suitable habitat features required for installation (e.g., a fallen log or rock pile). Each camera was positioned ~ 1.0 m above the ground and attached to a metal post, which faced a large horizontal log (or an equivalent rock pile) located between 1.5 and 3.0 m away from the camera. A lure canister containing ~ 500 g of raw chicken necks was pegged into the ground in front of the log. Cameras were aligned so that the camera’s upper detection zone covered the top of the log and the lower detection zone covered the lure canister as described in Henderson et al. (2021). Camera traps were deployed continuously from May to December 2020, and were serviced twice (August and November) to replace camera batteries, SD cards, and lures. The study was separated into three survey periods (May–June, August–September, and November–December), with each period commencing on the day that camera traps were serviced and lures refreshed, thus ensuring that the potential effect of declining lure age on quoll detectability remained consistent across survey periods (Henderson et al. 2022). To ensure temporal independence between survey periods and account for declining lure age, only the first 28 camera trap nights were included in data analysis.
Data analysis
All statistical analyses were performed in R version 4.2.0 (R Core Team 2022).
Naive occupancy
Raw detection data was used to determine naive occupancy for each vegetation type in each survey period. Naive occupancy expresses the proportion of camera sites where at least one quoll was detected, stratified by vegetation type. As not all the cameras operated for the full four weeks (due to battery depletion or camera malfunction), we only included detection data from cameras that were operational for at least 2 weeks.
Quoll activity across vegetation types
For each survey period, we used quoll activity (the number of independent quoll detections) in each vegetation type to infer habitat preference. Consecutive quoll detections on the same camera were considered independent detection events if image sequences were separated by more than 10 min, as described in Henderson et al. (2021). We used a chi-square goodness-of-fit test to compare the observed and expected a number of independent quoll detections across the four vegetation types. We then calculated the number of available camera nights for each vegetation type as a proportion of the total available camera nights across all vegetation types to determine the expected number of detections for each vegetation type. Available camera trap nights varied slightly between survey periods due to battery depletion or camera malfunction. Habitat preference was inferred where quolls were detected in vegetation types more frequently than expected.
Probability of occupancy across vegetation types
As raw detection data does not account for imperfect detection (MacKenzie et al. 2002), we also sought to infer habitat preference during each survey period using the probability of occupancy for each vegetation type. Occupancy probability was estimated using the single-species, single-season occupancy model in the “unmarked” package version 1.1.0 (Fiske and Chandler 2011). We created camera-specific detection histories by determining quoll presence (1) or absence (0) for each camera night using the package “camtrapR” version 2.0.3 (Niedballa et al. 2016). We defined a sampling “occasion” as a one-night period (24-h duration beginning at 12:00). Nights when cameras were inoperable (due to battery depletion or camera malfunction) for part or all of the 24-h period were recorded as “NA” in the detection history. We included vegetation type as a covariate on occupancy and lure age as a covariate on detection probability to account for the potential impact of lure efficiency over time (Henderson et al. 2022).
Temporal activity across vegetation types
To determine if habitat use differed temporally, we compared quoll temporal activity across vegetation types for each survey using the “overlap” package Version 0.3.2 (Ridout and Linkie 2009). To determine the extent of temporal activity overlap, we calculated the coefficient of overlap (Δ) whereby values range from 0 (no overlap) to 1 (complete overlap). Following Meredith and Ridout (2018), we used the Δ1 estimator because all sample sizes had < 5 detection events. We obtained 95% confidence intervals using 10,000 smoothed bootstrap samples (Rovero and Zimmermann 2016). We then used nonparametric kernel density curves to plot temporal activity profiles and tested for differences in temporal activity peaks using the nonparametric Mardia–Watson–Wheeler test for homogeneity within the ‘circular’ package Version 0.4–93 (Lund et al. 2017).
Results
Quoll activity across vegetation types
We recorded a total of 267 independent quoll detections from 3384 camera nights across the three survey periods. There was clear habitat flexibility, with quolls detected in all four vegetation types during each of the three survey periods. Naïve occupancy remained consistent between the May–June and August–September survey periods for all vegetation types, ranging from 50% in open grassland up to 89% in grassy woodland (Table 2a). However, naïve occupancy was low across all vegetation types during November–December, ranging from 20% in open grassland and up to 50% in both grassy woodland and wet sclerophyll forest (Table 2a).
Quoll activity differed significantly between vegetation types in all the three survey periods (all P < 0.05), although different habitats were preferred in different survey periods. During May–June, quolls were detected in dry sclerophyll forest more frequently than expected (X2 = 16.61, df = 3, P < 0.01) while grassy woodlands were preferred in both the August–September (X2 = 8.28, df = 3, P < 0.05) and November–December (X2 = 18.82, df = 3, P < 0.01) survey periods (Table 2b). In contrast, quolls were detected in wet sclerophyll forest less frequently than expected during both the May–June and November–December surveys while use of open grasslands was lower than expected in both the August–September and November–December survey periods (Table 2b).
Probability of occupancy across vegetation types
The probability of quoll occupancy did not reveal any significant difference in habitat use across vegetation types or between surveys as indicated by wide, overlapping confidence intervals (Fig. 2).
Temporal activity
There were no significant differences in activity peaks between each of the three forested vegetation types (grassy woodland, dry sclerophyll, and wet sclerophyll) for each survey (all P > 0.05). Therefore, we subsequently pooled these three vegetation types into a combined “Forest” category to compare temporal activity across the vegetation types. The quolls were primarily nocturnal (1800–0600 h) and exhibited a similar temporal activity between forest and open grassland during both May–June (Δ1 = 0.74, 95% CI: 0.53–0.83, Fig. 3a) and August–September (Δ1 = 0.83, 95% CI: 0.73–0.97, Fig. 3b). The nonparametric Mardia–Watson–Wheeler tests also showed no significant difference in activity peaks between the forest and open grassland habitats for both May–June (W = 4.30, P = 0.12) and August–September (W = 2.61, P = 0.27). The November–December survey was not compared due to the low number of quoll detections (n = 5) in the open grassland.
Discussion
Our study is the first to investigate habitat use by quolls in a fragmented landscape in mainland Australia. The raw detection data revealed some seasonal variations in habitat preferences, although quolls generally avoided open grassland and wet sclerophyll forest in preference for grassy woodland and dry sclerophyll forest. In contrast, occupancy modelling revealed broad habitat flexibility by quolls, with no specific habitat preference. Furthermore, quoll temporal activity remained similar between vegetation types and survey periods. Our results suggest that quoll habitat use in mainland Australia is likely more flexible than previously assumed and highlights the potential value of human-modified landscapes for quoll conservation. However, the conflicting results also highlight the inherent difficulties of reliably determining the habitat preference of cryptic carnivorous species such as quolls.
Our findings are consistent with those reported by Troy (2014) in Tasmania where quoll habitat use was flexible within fragmented agricultural landscapes. Troy (2014) suggested that an absence of foxes in Tasmania potentially explains the wide habitat tolerances of quolls observed in their study. In contrast, the quoll research in mainland Australia has predominately been conducted in intact forested landscapes, leading to an established notion that quolls are dependent on large, complex contiguous forests (Belcher 2000; Belcher and Darrant 2006; Mansergh 1983) because of the threat posed by foxes in more open landscapes (Long and Nelson 2010). However, Henderson et al. (2021) found that the presence of foxes at our study site did not appear to influence quoll activity, possibly due to low fox density. The broad habitat use by quolls observed in our current study possibly reflects a flexibility in habitat use under low fox densities, similar to the flexibility observed in the absence of foxes (Troy 2014). This suggests that quoll habitat use where foxes are absent or rare (e.g., in Tasmania and in our current study) might reflect the species’ preferred niche, while higher fox densities might restrict quoll habitat use to a realized niche of intact contiguous forest where the probability of survival is increased (Troy 2014). Further research is required to compare quoll habitat use in fragmented landscapes under different fox densities.
The observed habitat flexibility of quolls in our study might reflect prey availability across vegetation types as suggested by studies on other mesocarnivores in fragmented landscapes. For example, pine martens can benefit from supplemental food resources in fragmented habitats, and this can likely facilitate the tolerance of habitat loss up to a certain threshold (Caryl et al. 2012; Mortelliti and Boitani 2008). Similarly, forest-dependent leopard cats are able to utilize open agricultural plantations in Southeast Asia (Chua et al. 2016) due to the better prey catchability of abundant rodent pests (Lorica and Heaney 2013; Rajaratnam et al. 2007; Silmi et al. 2013). This could be similar for quolls in the open grassland, because the abundant prey species such as hares (Lepus europaeus) and northern brown bandicoots (Isoodon macrourus) (Glen and Dickman 2006a; Jarman et al. 2007) at our site were not limited to forested habitats but were also frequently detected on cameras in the open grassland where the catchability for these species might be greater than in forested areas. Further research is needed to investigate whether prey availability and catchability influence the habitat flexibility of quolls within fragmented landscapes.
The avoidance of open grasslands and preference for forest fragments were also consistent with observations of quolls in Tasmania (Troy 2014). Troy (2014) found a positive correlation between female quoll home range size and the proportion of pasture, and a negative correlation between home range size and eucalypt forests, suggesting that pastures contain insufficient resources to meet quoll resource demands while forests provide disproportionately more resources. In our study, the habitat preference might also reflect the resource availability for quolls within each vegetation type with the open grassland providing insufficient resources to induce preference. Within forested vegetation types, the strong preference for grassy woodland was surprising considering quolls in mainland Australia are typically associated with structurally complex contiguous forests such as sclerophyll forests or rainforests (Belcher 2000; Belcher and Darrant 2006; Mansergh 1983). At our site, the grassy woodlands support a mix of semi-arboreal prey such as brush-tailed possums (Trichosurus vulpecula) as well as ground-dwelling grassland species such as bandicoots and hares while also providing hollow-bearing trees or logs for shelter. These grassy woodlands could potentially act as a “buffer zone” between open grasslands and resource-rich sclerophyll forests to facilitate quoll foraging and movement while maintaining a low risk from predation. In contrast, quoll detections were less common in the wet sclerophyll forest, which includes a more structurally complex vegetation offering greater protective cover from other predators. It is possible that prey availability is lower in wet sclerophyll forest as it may provide prey with more cover from quolls compared to more open forests. Therefore, grassy woodlands and dry sclerophyll forests may provide a balance of sufficient den sites, cover from other predators, and increased functional availability of prey. However, further research is needed to understand the specific features that influence quoll preference within these vegetation types.
The observed differences in habitat preference between the survey periods suggest that seasonal variations in habitat use might be associated with the quoll's reproductive cycle. For example, the May–June survey coincides with the quoll’s annual breeding season (Belcher 2003) when transient males roam across large areas in search of females (Belcher and Darrant 2004). This possibly explains why quolls were detected more frequently than expected in the open grassland during this survey compared to the other survey periods when the open grassland was avoided. The aversion for the open grassland during August–September could reflect the need for females to remain within the protection of forested habitats, where they are less vulnerable to predation while they are carrying pouch young during this time of the year (Körtner et al. 2019). A similar seasonal variation in habitat use has been observed for raccoons in Canada, which are known to forage in open agricultural environments but prefer forested habitats when rearing young (Beasley et al. 2007a). In addition, the November–December survey period coincides with juvenile quolls initially emerging from their natal dens and becoming independent (Andrew 2005), potentially avoiding the open grassland where they are more exposed to other predators. Surprisingly, quolls did not prefer wet sclerophyll forest, which is likely to provide more cover from other predators. In fact, quoll activity was relatively lower in wet sclerophyll in both the May–June and November–December survey periods compared to the other forested vegetation types. This may instead reflect seasonal variations in prey availability or catchability within wet sclerophyll forests. Future studies should investigate quoll habitat use during different times of the year and across multiple years to determine if seasonal variation in habitat use is consistent with our findings and hypotheses.
The broad habitat use of quolls and a strong preference for forest fragments suggest that quolls are likely traversing open grasslands to access these forest fragments. In Tasmania, quolls used small, isolated vegetation patches as “stepping stones” between larger forest patches (Troy 2014). Our study site similarly comprised many small clusters of paddock trees or isolated exotic shrubs, which potentially act as refugia within the open grasslands that stretch between the larger forested vegetation types. These vegetation patches may be important for facilitating connectivity within the landscape and provide additional resources for quolls such as den sites or prey. Further research is needed to ascertain fine-scale movements of quolls throughout this fragmented landscape to understand the importance of these isolated vegetation patches so that conservation strategies can ensure the protection of critical vegetation within the fragmented matrix.
Our occupancy modelling analysis was unable to discern any significant difference in habitat use across vegetation types. Estimates derived using an occupancy modelling approach are generally considered more robust as they account for imperfect detection data (MacKenzie et al. 2006). Accordingly, it is possible that our occupancy modelling estimates accurately reflect the habitat use by quolls and that there is actually no habitat preference for the quolls at this site. The observed preference inferred from raw detection data might simply reflect a detection bias as a result of imperfect detection, although this is uncertain. Conversely, the large and overlapping confidence intervals around our occupancy estimates could suggest the study design or sample size was inadequate to determine any habitat preference using occupancy, which is typically a data-hungry analysis (Jha et al. 2022). This could be due to the cryptic nature of quolls, whereby the probability of occupancy may be high, but the probability of detection is very low, resulting in imprecise estimates. Greatly increasing the number of sampling occasions can better inform the probability of detection (MacKenzie 2005). Additionally, the precision of occupancy estimates can be improved by increasing the number of sites (MacKenzie et al. 2006). Such studies may, however, be limited by financial or logistical constraints. Therefore, a trade-off between sufficient spatial replicates and sampling occasions is needed to achieve a practical survey effort. This highlights the potential limitation of using occupancy to study low-density and wide-ranging carnivores such as quolls, which may require a much greater survey effort than what is feasible.
Information on quoll habitat use in fragmented landscapes can inform appropriate conservation and management strategies by identifying important habitat to be retained and protected at the landscape level. The quolls in our study exhibited a strong preference for grassy woodlands and dry sclerophyll forests with varying degrees of preference at different times of the year. The quolls also demonstrated greater flexibility in habitat use than previously assumed in mainland Australia, indicating some adaptability to fragmented landscapes. However, due to the limitations of the analyses used, our interpretations should be treated cautiously. Nevertheless, the ability for quolls to persist in this fragmented landscape highlights our incomplete understanding of the importance of fragmented habitat for endangered predator species such as quolls. While habitat loss is often considered the greatest threat to biodiversity loss (Rands et al. 2010), the extent to which habitat fragmentation affects biodiversity remains contentious (Fahrig 2017; Fletcher et al. 2018). Quolls are generalist carnivores and may indeed benefit from habitat fragmentation, provided other larger carnivores such as foxes remain absent or at low densities. Future research should investigate specific factors that might influence quoll habitat preference such as predator densities, prey availability, and seasonal behavior. In addition, future quoll research should replicate our study in other fragmented landscapes and attempt to incorporate greater survey effort to ensure adequate data is obtained to infer habitat preferences. Continued research will improve our understanding of quoll ecology outside of contiguous forested areas and assist with quoll conservation in fragmented landscapes.
Data availability
The data used in this paper are available from the corresponding author upon reasonable request.
References
Lund U, Agostinelli C, Arai H, Gagliardi A, Portugues EG, Giunchi D, Irrison J, Pocernich M, Rotolo F (2017) Package ‘circular’. https://cran.r-project.org/web/packages/circular/circular.pdf. Accessed March 2021.
Andersen GE, Johnson CN, Barmuta LA, Jones ME (2017) Use of anthropogenic linear features by two medium-sized carnivores in reserved and agricultural landscapes. Sci Rep 7(1):11624
Andrew D (2005) Ecology of the tiger quoll Dasyurus maculatus maculatus in coastal New South Wales. MSc Thesis, School of Biological Sciences, University of Wollongong, 2005. http://ro.uow.edu.au/theses/686
Bateman PW, Fleming PA (2012) Big city life: carnivores in urban environments. J Zool 287(1):1–23
Beasley J, Devault T, Retamosa M, Rhodes O (2007) A hierarchical analysis of habitat selection by raccoons in Northern Indiana. J Wildl Manag 71(4):1125–1133
Beasley J, Devault T, Rhodes O (2007) Home-range attributes of raccoons in a fragmented agricultural region of northern Indiana. J Wildl Manag 71(3):844–850
Belcher CA (2000) Ecology of the tiger quoll Dasyurus maculatus in southeast Australia. Deakin University, Geelong, Australia
Belcher CA (2003) Demographics of tiger quoll (Dasyurus maculatus maculatus) populations in south-eastern Australia. Aust J Zool 51(6):611–626
Belcher CA, Darrant JP (2004) Home range and spatial organization of the marsupial carnivore, Dasyurus maculatus maculatus (Marsupialia: Dasyuridae) in south-eastern Australia. J Zool 262(3):271–280
Belcher CA, Darrant JP (2006) Habitat use by tiger quoll (Dasyurus maculatus) (Marsupialia: Dasyuridae) in south-eastern Australia. J Zool 269(2):183–190
Belcher CA (2004) The largest surviving marsupial carnivore on mainland Australia: the tiger or spotted-tailed quoll Dasyurus maculatus, a nationally threatened, forest-dependent species. In ‘Conservation of Australia’s Forest Fauna’. (Ed. D. Lunney.) pp. 612–623. (Royal Zoological Society of New South Wales: Sydney.)
Bino G, Dolev A, Yosha D, Guter A, King R, Saltz D, Kark S (2010) Abrupt spatial and numerical responses of overabundant foxes to a reduction in anthropogenic resources. J Appl Ecol 47(6):1262–1271
Caryl FM, Quine CP, Park KJ (2012) Martens in the matrix: the importance of nonforested habitats for forest carnivores in fragmented landscapes. J Mammal 93(2):464–474
Catling PC, Burt RJ (1995) Why are red foxes absent from some eucalypt forests in eastern New South Wales? Wildl Res 22(4):535–545
Catling PC, Burt RJ, Forrester RI (2002) Models of the distribution and abundance of ground-dwelling mammals in the eucalypt forests of north-eastern New South Wales in relation to environmental variables. Wildl Res 29(3):313–322
Chapron G, Kaczensky P, Linnell J, von Arx M, Huber D, Andrén H, López-Bao JV, Adamec M, Álvares F, Anders O, Balciauskas L, Balys V, Bedő P, Bego F, Blanco J, Breitenmoser U, Brøseth H, Bufka L, Bunikyte R, Boitani L (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Sci 346:1517–1519
Chua MA, Sivasothi N, Meier R (2016) Population density, spatiotemporal use and diet of the leopard cat (Prionailurus bengalensis) in a human-modified succession forest landscape of Singapore. Mamm Res 61(2):99–108
Claridge AW, Paull D, Dawson J, Mifsud G, Murray AJ, Poore R, Saxon MJ (2005) Home range of the spotted-tailed quoll (Dasyurus maculatus), a marsupial carnivore, in a rainshadow woodland. Wildl Res 32(1):7–14
Crooks KR (2002) Relative sensitivities of mammalian carnivores to habitat fragmentation. Conserv Biol 16(2):488–502
Crooks K, Burdett CL, Theobald DM, Rondinini C, Boitani L (2011) Global patterns of fragmentation and connectivity of mammalian carnivore habitat. Phil Trans R Soc B: Biol Sci 366(1578):2642–2651
ESRI (2015) ArcGIS 10.4.1 for desktop. In ‘Esri Inc.’ https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview)
Fahrig L (2017) Ecological responses to habitat fragmentation per se. Annu Rev Ecol Evol Syst 48(1):1–23
Farris ZJ, Golden CD, Karpanty S, Murphy A, Stauffer D, Ratelolahy F, Andrianjakarivelo V, Holmes CM, Kelly MJ (2015) Hunting, exotic carnivores, and habitat loss: anthropogenic effects on a native carnivore community, Madagascar. PLOS ONE 10(9):0136456
Fiske I, Chandler R (2011) unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance. Journal of Statistical Software, 43(10):1–23
Fletcher RJ, Didham RK, Banks-Leite C, Barlow J, Ewers RM, Rosindell J, Holt RD, Gonzalez A, Pardini R, Damschen EI, Melo FPL, Ries L, Prevedello JA, Tscharntke T, Laurance WF, Lovejoy T, Haddad NM (2018) Is habitat fragmentation good for biodiversity? Biol Cons 226:9–15
Gehring TM, Swihart RK (2003) Body size, niche breadth, and ecologically scaled responses to habitat fragmentation: mammalian predators in an agricultural landscape. Biol conserv 109(2):283–295
Gehring TM, Swihart RK (2004) Home range and movements of long-tailed weasels in a landscape fragmented by agriculture. J Mammal 85(1):79–86
Gittleman, JL, Funk, SM, MacDonald, DW, Wayne, RK (Eds.) (2001) Carnivore conservation (Vol. 5). Cambridge: Cambridge University Press.
Glen AS, Dickman CR (2005) Complex interactions among mammalian carnivores in Australia, and their implications for wildlife management. Biol Rev 80(3):387–401
Glen AS, Dickman CR (2006) Diet of the spotted-tailed quoll (Dasyurus maculatus) in eastern Australia: effects of season, sex and size. J Zool 269(2):241–248
Glen AS, Dickman CR (2006) Home range, denning behaviour and microhabitat use of the carnivorous marsupial Dasyurus maculatus in eastern Australia. J Zool 268(4):347–354
Glen AS, Dickman CR (2008) Niche overlap between marsupial and eutherian carnivores: does competition threaten the endangered spotted-tailed quoll? J Appl Ecol 45(2):700–707
Glen AS, Pennay M, Dickman CR, Wintle BA, Firestone KB (2011) Diets of sympatric native and introduced carnivores in the Barrington Tops, eastern Australia. Austral Ecol 36(3):290–296
Haag T, Santos AS, Sana DA, Morato RG, Cullen L Jr, Crawshaw PG Jr, De Angelo C, Di Bitetti MS, Salzano FM, Eizirik E (2010) The effect of habitat fragmentation on the genetic structure of a top predator: loss of diversity and high differentiation among remnant populations of Atlantic forest jaguars (Panthera onca). Mol Ecol 19(22):4906–4921
Hamer RP, Andersen GE, Hradsky BA, Troy SN, Gardiner RZ, Johnson CN, Jones ME (2021) Differing effects of productivity on home-range size and population density of a native and an invasive mammalian carnivore. Wildl Res 49(1):158–168
Henderson T, Fancourt BA, Rajaratnam R, Vernes K, Ballard G (2021) Spatial and temporal interactions between endangered spotted-tailed quolls and introduced red foxes in a fragmented landscape. J Zool 315:276–287
Henderson T, Fancourt BA, Ballard G (2022) The importance of species-specific survey designs: prey camera trap surveys significantly underestimate the detectability of endangered spotted-tailed quolls. Aust Mammal 44(3):380–386
Henner CM, Chamberlain MJ, Leopold BD, Burger JR W (2004) A multi-resolution assessment of raccoon den selection. J Wildl Manag 68(1):179–187
Henderson T, Fancourt BA, Rajaratnam R, Vernes K, Ballard G (2022) Density estimates reveal that fragmented landscapes provide important habitat for conserving an endangered mesopredator, the spotted-tailed quoll. Sci Rep12:12688
Hradsky BA, Robley A, Alexander R, Ritchie EG, York A, Di Stefano J (2017) Human-modified habitats facilitate forest-dwelling populations of an invasive predator, Vulpes vulpes. Sci Rep 7:12291
Jarman PJ, Allen LR, Boschma DJ, Green SW (2007) Scat contents of the spotted-tailed quoll Dasyurus maculatus in the New England gorges, north-eastern New South Wales Australian Journal of Zoology. Aust J Zool 55(1):63–72
Jha A, J P, Nameer PO (2022) Contrasting occupancy models with presence-only models: does accounting for detection lead to better predictions? Ecol Model 472:110105
Jones ME, Rose RK, Burnett S (2001) Dasyurus maculatus. Mamm Species 676:1–9
Jones ME, Burnett S, Claridge AW, Fancourt B, Kortner G, Morris K, Peacock D, Troy S, Woinarski J (2014) Australia’s surviving marsupial carnivores: threats and conservation. Collingwood, Australia, CSIRO Publishing
Jones ME, Bain GC, Hamer RP, Proft KM, Gardiner RZ, Dixon KJ, Kittipalawattanapol K, Zepeda de Alba AL, Ranyard CE, Munks SA, Barmuta LA, Burridge CP, Johnson CN, Davidson NJ (2021) Research supporting restoration aiming to make a fragmented landscape ‘functional’ for native wildlife. Ecol Manag Restor 22(S2):65–74
Körtner G, Gresser S, Mott B, Tamayo B, Pisanu P, Bayne P, Harden R (2004) Population structure, turnover and movement of spotted-tailed quolls on the New England Tablelands. Wildl Res 31(5):475–484
Körtner G, Claridge A, Ballard G (2019) Denning behaviour of female spotted-tailed quolls during the breeding season. Aust J Zool 67(3):145–152
Kremen C, Merenlender AM (2018) Landscapes that work for biodiversity and people. Sci 362(6412):eaau6020
Lindenmayer DB, Fischer J (2013) Habitat fragmentation and landscape change: an ecological and conservation synthesis. Washington: Island Press
Long K, Nelson J (2010) National recovery plan for the spotted-tailed quoll Dasyurus maculatus. Vic Dep Sustain Environ. Available at: Available at: http://www.environment.gov.au/system/files/resources/2343110b-d2b4-4a1f-b66e-ddfae63c4aa6/files/national-recovery-plan-spotted-tailed-quoll.pdf
Lorica M, Heaney L (2013) Survival of a native mammalian carnivore, the leopard cat Prionailurus bengalensis Kerr, 1792 (Carnivora: Felidae), in an agricultural landscape on an oceanic Philippine island. J Threat Taxa, 5(10):4451–4460
MacKenzie DI (2005) What are the issues with presence-absence data for wildlife managers? J Wildl Manag 69(3):849–860
MacKenzie D, Nichols J, Lachman G, Droege S, Royle JA, Langtimm C (2002) Estimating site occupancy rates when detection probabilities are less than one. Ecol 83:2248–2255
MacKenzie DI, Nichols JD, Royle JA, Pollock KH, Bailey LL, Hines JE (2006) Occupancy estimation and modeling : inferring patterns and dynamics of species occurrence. Burlington, MA, Elsevier/Academic Press
Mansergh I (1983) status, distribution and abundance of Dasyurus maculatus (Tiger Quoll) in Australia, with particular reference to Victoria [largest marsupial carnivore]. Aust Zool 21:109–122
Maxwell S, Burbidge A, Morris K (1996) Spotted-tailed Quoll (SE mainland and Tas); recovery outline. In ‘The Action Plan for Australian Marsupials and Monotremes. 85–87. Environment Australia, Canberra, ACT
McLean CM, Vårhammar A, Mikac KM (2015) Use of motion-activated remote cameras to detect the endangered spotted-tailed quoll (Dasyurus maculatus): results from a pilot study. Aust Mammal 37(1):113–115
Meredith M, Ridout M (2018) Overview of the overlap package. R Project. 1–9. https://cran.rproject.org/web/packages/overlap/vignettes/overlap.pdf. Accessed March 2022
Mergey M, Helder R, Roeder JJ (2011) Effect of forest fragmentation on space-use patterns in the European pine marten (Martes martes). J Mammal 92(2):328–335
Mergey M, Larroque J, Ruette S, Vandel JM, Helder R, Queney G, Devillard S (2012) Linking habitat characteristics with genetic diversity of the European pine marten (Martes martes) in France. Eur J Wildl Res 58(6):909–922
Mortelliti A, Boitani L (2008) Interaction of food resources and landscape structure in determining the probability of patch use by carnivores in fragmented landscapes. Landscape Ecol 23(3):285–298
Niedballa J, Sollmann R, Courtiol A, Wilting A (2016) camtrapR: an R package for efficient camera trap data management. Methods Ecol Evol 7(12):1457–1462
Pereboom V, Mergey M, Villerette N, Helder R, Gerard JF, Lode T (2008) Movement patterns, habitat selection, and corridor use of a typical woodland-dweller species, the European pine marten (Martes martes), in fragmented landscape. Can J Zool 86(9):983–991
R Core Team (2022) R: A language and environment for statistical computing. In ’ ’. Vienna, Austria, R Foundation for Statistical Computing
Rajaratnam R, Sunquist M, Rajaratnam L, Ambu L (2007) Diet and habitat selection of the leopard cat (Prionailurus bengalensis borneoensis) in an agricultural landscape in Sabah, Malaysian Borneo. J Trop Ecol 23(2):209–217 ([In English])
Rands MR, Adams WM, Bennun L, Butchart SH, Clements A, Coomes D, Entwistle A, Hodge I, Kapos V, Scharlemann JP (2010) Biodiversity conservation: challenges beyond 2010. Sci 329(5997):1298–1303
Remonti L, Balestrieri A, Ruiz-González A, Gómez-Moliner BJ, Capelli E, Prigioni C (2012) Intraguild dietary overlap and its possible relationship to the coexistence of mesocarnivores in intensive agricultural habitats. Popul Ecol 54(4):521–532
Ridout MS, Linkie M (2009) Estimating overlap of daily activity patterns from camera trap data. J Agric Biol Environ Stat 14(3):322–337
Ripple WJ, Estes JA, Beschta RL, Wilmers CC, Ritchie EG, Hebblewhite M, Berger J, Elmhagen B, Letnic M, Nelson MP, Schmitz OJ, Smith DW, Wallach AD, Wirsing AJ (2014) Status and ecological effects of the world’s largest carnivores. Sci 343(6167).
Ritchie H, Roser M (2021) Forests and deforestation. Published online at OurWorldInData.org. Retrieved from: https://ourworldindata.org/forests-and-deforestation. Accessed October 2022.
Rovero F, Zimmermann F (2016) Camera trapping for wildlife research. Data in the wild. Exeter: Pelagic Publishing
Shapira I, Sultan H, Shanas U (2008) Agricultural farming alters predator-prey interactions in nearby natural habitats. Anim Conserv 11(1):1–8
Silmi M, Mislan M, Anggara S, Dahlen B (2013) Using leopard cats (Prionailurus bengalensis) as biological pest control of rats in a palm oil plantation. J Indones Nat Hist 1(1):31–36
Schüttler E, Klenke R, Galuppo S, Castro RA, Bonacic C, Laker J, Henle K (2017) Habitat use and sensitivity to fragmentation in America’s smallest wildcat. Mammalian Biology 86:1–8
Swihart RK, Gehring TM, Kolozsvary MB, Nupp TE (2003) Responses of ‘resistant’ vertebrates to habitat loss and fragmentation: the importance of niche breadth and range boundaries. Divers Distrib 9(1):1–18
Troy SN (2014) Spatial ecology of the Tasmanian spotted-tailed quoll (Doctoral dissertation, University of Tasmania)
Weber D, Roth T, Tesini C, Thiel D (2018) Widespread distribution of Pine martens (Martes martes) in a fragmented suburban landscape. Mamm Res 63(3):349–356
Acknowledgements
We thank Joshua Van der Eyk, Heath Milne, Brent Klohk, and Conor Nest for the assistance during fieldwork. Access to the study site was provided by Liddell Coal Operations Pty Limited. We also thank the owner of the adjacent private land for allowing us access to their property.
Funding
Liddell Coal Operations Pty Limited funded the fieldwork costs and equipment. The Holsworth Wildlife Research Endowment supported the funding for the additional equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The approval for the work was provided by the University of New England Animal Ethics Committee (AEC19-044).
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Andrzej Zalewski.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Henderson, T., Fancourt, B.A., Rajaratnam, R. et al. Habitat use by the endangered spotted-tailed quoll in a fragmented landscape. Mamm Res 68, 93–103 (2023). https://doi.org/10.1007/s13364-022-00660-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-022-00660-4