Abstract
The specific positions of carbon–carbon double bond(s) within an unsaturated fatty acid exert a significant effect on the physical and chemical properties of the lipid that ultimately inform its biological function(s). Contemporary liquid chromatography–mass spectrometry (MS) strategies based on electrospray ionization coupled to tandem MS can easily detect fatty acyl lipids but generally cannot reveal those specific site(s) of unsaturation. Herein, we describe a novel and versatile workflow whereby fatty acids are first converted to fixed charge N-(4-aminomethylphenyl)pyridinium (AMPP) derivatives and subsequently subjected to ozone-induced dissociation (OzID) on a modified triple quadrupole mass spectrometer. The AMPP modification enhances the detection of fatty acids introduced by direct infusion. Fragmentation of the derivatized fatty acids also provides diagnostic fragment ions upon collision-induced dissociation that can be targeted in precursor ion scans to subsequently trigger OzID analyses in an automated data-dependent workflow. It is these OzID analyses that provide unambiguous assignment of carbon–carbon double bond locations in the AMPP-derivatized fatty acids. The performance of this analysis pipeline is assessed in profiling the patterns of unsaturation in fatty acids within the complex biological secretion vernix caseosa. This analysis uncovers significant isomeric diversity within the fatty acid pool of this sample, including a number of hitherto unreported double bond positional isomers that hint at the activity of potentially new metabolic pathways.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gas chromatography (GC) of fatty acids (FAs) derivatized as methyl esters (FAMEs) is considered the “gold standard” for fatty acid analysis, principally owing to the speed and resolution of the chromatography [1, 2]. GC is most often coupled to electron ionization (EI) mass spectrometry (MS), which generally enables sensitive and selective identification of the fatty acids as the corresponding methyl ester derivatives. However, in the absence of pure standards for retention time alignment, GC-MS is often unable to differentiate structurally related lipids (e.g., monounsaturated fatty acids that differ only in the position or stereochemistry of the double bond), due to similarities in the resulting EI spectra [2]. This limitation can be at least partially overcome by the use of alternate derivatization strategies, such as dimethyloxazoline or picolinyl esters, which yield diagnostic spectra [3,4,5].
In parallel, electrospray ionization (ESI) MS has emerged as the pre-eminent technique for classification of complex lipid classes (e.g., phospholipids, triacylglycerols). While they are powerful for complex lipids, these protocols are less effective for fatty acid analysis as they can typically provide only scant structural details beyond the total number of carbons and degrees of unsaturation in the acyl chain [6,7,8]. This is mostly because low-energy collision-induced dissociation (CID) of fatty acid anions (either non-esterified fatty acids or hydrolyzed complex lipids) yields only non-specific product ions arising from dehydration or decarboxylation. Positions of unsaturation or other chain modifications cannot typically be assigned [9] without the use of alternative tandem mass spectrometry strategies (e.g., high-energy CID [10] or derivatization of the double bonds by the Paternò–Büchi reaction followed by low-energy CID [11,12,13]). This problem is not readily ameliorated in hyphenated liquid chromatography (LC)–MS workflows, as the inherently lower chromatographic resolution—compared with GC—commonly results in co-elution of structurally related or isomeric fatty acids [2]. Additionally, the sensitivity of fatty acid analysis by ESI in the negative ion mode is typically compromised by the use of acidic mobile phases in reversed-phase HPLC [14].
To circumvent these limitations, novel charge-switching chemical derivatives have been developed for fatty acid analysis [15], including N-(4-aminomethylphenyl)pyridinium (AMPP) salts [14, 16]. These derivatives are optimized for ESI-MS/MS and deliver benefits in three areas, namely, (i) up to 20-fold improvement in MS detection sensitivity due to the fixed positive charge [14, 17], (ii) formation of diagnostic product ions upon CID derived from charge-remote fragmentation, and (iii) compatibility with higher molecular weight acids that cannot be analyzed by conventional GC-MS [18,19,20]. For example, AMPP derivatives of individual fatty acids have been shown to yield unique CID spectra that can be used to identify positions of unsaturation or methyl chain branching [16, 17, 20, 21]. However, one disadvantage in the application of AMPP derivatization in direct infusion (or “shotgun”) workflows is that in complex mixtures, the resulting CID spectra represent a composite of the fragmentation of all isobaric and isomeric species which can be complex to deconvolute. This problem is particularly acute for isomeric fatty acids that differ only in the position(s) of unsaturation and are commonly present as mixtures in biological extracts [6].
Ozone-induced dissociation (OzID) [22, 23] is an ion activation technology that exploits the gas-phase reaction between mass-selected unsaturated lipid ions and ozone inside a mass spectrometer and yield spectra that readily identify sites of unsaturation, even in complex isomeric mixtures. Here we describe a new and versatile OzID implementation specifically for analysis of fatty acids as AMPP derivatives using a facile, direct infusion “shotgun” approach on a triple quadrupole mass spectrometer, using an AMPP-specific precursor ion scan to generate a dynamic target list for subsequent OzID analysis of all unsaturated fatty acid (FA) AMPP derivatives. We demonstrate this workflow to profile hydrolyzed fatty acids from a triacylglycerol fraction of vernix caseosa (VC), a heterogeneous biofilm that covers the skin of human fetuses and newborns and plays an important role in preventing dehydration and bacterial infection after birth [24]. VC is a complex mixture consisting of water-containing corneocytes embedded in a lipid matrix, with the lipid portion deriving from both sebaceous gland secretions and the epidermis. Previous work has shown that the lipid profile of VC differs between males and females and may also vary with the age, health, and development of the fetus [25]. The lipid portion is extremely complex and a topic of ongoing research, but is known to contain wax esters, cholesteryl esters, diol diesters, and triacylglycerols, amongst other classes [25,26,27,28,29,30]. Within these complex lipids, approximately 35% contain at least one double bond [29]. Highlighting the complexity of the VC lipidome, Hauff and Vetter identified 133 unique fatty acids from VC, including 65 mono- or poly-unsaturates ranging from FA 14:1 (6 isomers) to 20:3 (4 isomers) [27]. However, even this comprehensive GC-MS analysis was not able to identify specific double bond positions or branching locations within the various isomeric contributors. The inherent lipid complexity of VC and the limited understanding of the unsaturated lipids from this source made it an ideal proving ground for the AMPP–OzID method development. This rapid analysis reveals the presence of a range of double bond positional isomers in this fatty acid pool that are not readily identified by conventional GC-MS analysis.
Experimental Methods
Lipid Nomenclature
The nomenclature used here is based on literature recommendations [31, 32]. The sites of unsaturation are generally indicated using the “n-x” nomenclature, where n denotes the number of carbon atoms in the acyl chain and subtraction of x provides the location of the double bond relative to the methyl terminus [33]. For example, a fatty acid labelled as “FA 18:3(n-3, n-6, n-9)” has an 18-carbon chain length, with three carbon–carbon double bonds located at 3, 6, and 9 carbons away from the terminus (tail) of the acyl chain. In some instances, it is informative to indicate the location of the double bond with respect to the carboxylate moiety. In such cases, we employ the Δy nomenclature, where y indicates the position of unsaturation counted from the carboxylate group and not the terminus/tail.
Materials
All solvents for analysis were of HPLC grade or higher. AMPP derivatization was carried out using an AMP+ Mass Spectrometry Kit (Cayman Chemical, Ann Arbor, MI). A commercial stock solution of 37 fatty acid methyl esters was purchased from Restek (catalogue number 35077; Restek, Bellefonte, PA).
Preparation of Fatty Acid Derivatives
A triacylglycerol (TG) fraction from VC was collected and purified by thin-layer chromatography as previously described [28, 34]. Briefly, lipids were separated on glass plates (60 × 76 mm) coated with silica gel (60 G for thin-layer chromatography, Merck, Darmstadt, Germany) using hexane:diethyl ether (93:7 v/v) mobile phase. The zones were visualized under UV light after spraying with rhodamine 6G (0.05% in ethanol). Silica gel with TGs (RF = 0.16–0.25) was scraped off the plates, and the lipids were extracted with 10 mL of freshly distilled diethyl ether. The solvent was evaporated under a nitrogen stream. A total of 200 μg of this TG fraction was hydrolyzed to free fatty acids by dissolving in 1:1 methanol:tetrabutylammonium hydroxide (40% w/w in H2O) and heating at 75 °C for 2 h. The resulting mixture was extracted in equal volumes of water and hexane. The organic fraction was retained and dried under nitrogen. The hydrolyzed fatty acids were then functionalized with the AMPP reagent, following the method of Bollinger et al. [14, 17]. Briefly, 20 μL of 4:1 acetonitrile:dimethylformamide was added to the hydrolyzed TG fraction prepared above, followed by 20 μL 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC; 640 mM in water), 10 μL hydroxybenzotriazole (20 mM in 99:1 acetonitrile:dimethylformamide), and 30 μL of AMPP coupling reagent (20 mM in acetonitrile). The resulting solution was vortexed, sealed, and heated at 60 °C for 30 min. After cooling to room temperature, 1 mL of methyl tert-butyl ether (MTBE) and 1 mL of water were added. The organic upper layer was then dried under N2 and reconstituted in 100 μL of 1:1 (v/v) methanol/chloroform for ESI-MS. A commercial stock solution of 37 fatty acid methyl esters was hydrolyzed and derivatized (as above) to provide a test set of AMPP fatty acyl standards for comparison. A 100-μL aliquot was hydrolyzed to free fatty acids and converted to AMPP derivatives using the same protocols as above. Full experimental details for the derivatization of the FAME standard mix are reported in the Supporting Information.
For GC-MS analysis, a separate aliquot of the standard FAME mixture in dichloromethane was diluted 10-fold in hexane for GC-MS analysis. Similarly, 0.175 mg of the dried TG fraction from VC was hydrolyzed and esterified to FAMEs by adding 50 μL MTBE (containing 0.01% butylated hydroxytoluene) and 10 μL trimethylsulfonium hydroxide and vortex-mixing for 20 s.
Electrospray Ionization Mass Spectrometry
AMPP derivatized fatty acids (AMPP-FAs) were diluted 100-fold in methanol and infused directly into the electrospray ionization source of a QTRAP 6500 hybrid triple quadrupole/linear ion trap mass spectrometer (SCIEX, Concord, ON, Canada). Typical source parameters used were a spray voltage of +5 kV, source heater temperature of 100 °C, and both source gasses set to 15 (arb.). A data-dependent batch method was used to target AMPP-FAs via a precursor ion survey scan (collision energy = 65 V) for the charged fragments of the AMPP head group (m/z 169 and 183) [14, 17]. The CID fragmentation efficiency to the m/z 183 product ions for the derivatized fatty acids is dependent on mass and the degree of unsaturation in the lipid (See Supporting Information, Figure S1). For the qualitative analysis presented here, a compromise collision energy of 65 V was selected but for quantitative workflows (beyond the scope of this study), a ramped CID would be recommended. AMPP-FA ions identified in the precursor ion scans were subsequently targeted for ozone-induced dissociation (OzID) to determine sites of unsaturation.
Ozone (ca. 18% in oxygen) was produced by an ozone generator (Titan30 UHC; Absolute Ozone, Edmonton, Canada) and introduced into the collision cell (q2) of the QTRAP 6500 via a PEEKsil restriction in place of the native CAD gas supply. Ozonolysis of mass-selected target ions was conducted in q2 of the QTRAP 6500 using a modified instrument method to enact ion trapping (ttrap = 2 s) in this region [23, 35, 36]. Data were processed using a bespoke data extraction plugin for PeakView (v2.2; SCIEX). This routine extracts peak areas and intensities for the survey and data-dependent mass spectra, along with the precursor ion for the MS2 experiments. Scans with precursor ions within ± 0.3 Da of one another were summed prior to extraction. This data set was then processed using Python to reconstruct pseudo-neutral loss scans for the characteristic OzID transitions [37].
A high-level overview of the mass spectrometry workflow, including extraction and derivatization, is provided as Figure S2 of the Supporting Information.
Gas Chromatography–Mass Spectrometry
Fatty acid methyl ester samples were analyzed with a gas chromatograph coupled to a mass spectrometer (GC-MS, TQ8040; Shimadzu, Kyoto, Japan). This analysis utilized an RTX-2330 capillary column (60 m × 0.25 mm, film thickness 0.20 μm; Restek, Bellefonte, PA, USA), and the electron ionization was set at 70 eV. Conditions for the analysis of FAMEs were as follows: carrier gas, He 2.6 mL/min; 22:1 split ratio, injection volume 1 μL; injector temperature 220 °C; thermal gradient 150 to 170 °C at 3 °C/min, then 170 to 250 °C at 2 °C/min, and temperature held for 3.5 min. The data were acquired with Q3 scan mode from m/z 50–650. For data collection, the MS spectra were recorded from 4 to 50 min. Where possible, retention times were aligned against a standard mixture of 37 FAMEs in dichloromethane (Food Industry FAME Mix, Restek, catalogue number 35077), diluted 10-fold in n-hexane. The resulting GC-MS data were visualized using the Shimadzu GCMS Postrun software (v4.45 SP1), and mass spectra were matched against the NIST GC-MS Library (v14).
Results
The utility of charge-switch derivatization for OzID was initially explored using fatty acids obtained from a commercially available FAME mixture. This calibration standard presents a means for testing the workflow with a broad range of fatty acyl chain lengths and double bond positions. Figure 1(a) shows a m/z 183 precursor ion spectrum diagnostic for the AMPP functionality in positive ion mode (C12H11N2+). For comparison, a GC-MS total ion chromatogram of the original FAME mixture is presented in Figure 1(b). In both cases, peaks are observed that correspond to C14–C30 acyl chain lengths.
Analysis of the commercially available calibration mixture comprised of 37 fatty acids: (a) AMPP derivatization and subsequent precursor ion scan for m/z 183; (b) GC-MS chromatogram showing the FAMEs present in the original mixture. Butylated hydroxytoluene, present as an antioxidant, is indicated by asterisk in the GC trace. Complete assignment of the GC-MS trace is provided in Table S1 of the Supporting Information
Mass selection and reaction of AMPP-FA 16:1 (m/z 421) with ozone for 1 s produces the spectrum shown in Figure 2(a). The single pair of OzID product ions (m/z 339 and 355, neutral losses of 82 and 66 Da, respectively) reveals that this monounsaturated fatty acyl exists in the standard mixture as a single isomer with the site of unsaturation in the n-7 position (see Supporting Information Figure S3). The observation of a single AMPP-FA 16:1 composition is consistent with the GC-MS analysis which gives only a single peak for FAME 16:1 at 18.4 min (Figure 1(b)). The advantage of the OzID analysis in Figure 2(a) lies in the explicit definition of the site of unsaturation which cannot be derived from the corresponding electron ionization mass spectra underlying the GC trace (data not shown).
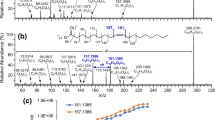
OzID spectra of (a) AMPP-FA 16:1 (m/z 421), displaying OzID fragments characteristic of a double bond at n-7, and (b) AMPP-FA 18:3 (m/z 445). Red lines indicate peaks diagnostic for polyunsaturated FA 18:3(n-3, n-6, n-9), and black lines indicate peaks characteristic of FA 18:3(n-6, n-9, n-12). (c) CID mass spectrum of AMPP-FA 18:3 (m/z 445)
In contrast, the polyunsaturated FAME 18:3 is present as a mixture of two double bond positional isomers, eluting from the GC at 14.1 and 14.9 min (Figure 1(b) and Table S1). After derivatization, both AMPP-FA 18:3 isomers appear at m/z 445 in the precursor ion scan. Mass selection of m/z 445 and subsequent trapping in the presence of ozone for 1 s result in the OzID mass spectrum presented in Figure 2(b). Pairs of diagnostic OzID ions, each separated by 16 Da, confirm the double bond position(s) to be 18:3(n-3, n-6, n-9) and 18:3(n-6, n-9, n-12), consistent with α-linolenic acid and γ-linolenic acid, respectively. The relative abundances of the diagnostic product ions are greater than previously reported for ozonolysis of sodiated adducts of methylene interrupted polyunsaturated FAMEs [38]. In contrast to the OzID spectrum, the low-energy CID spectrum of m/z 445 (Figure 2(c)) is highly complex, making deconvolution and identification of the two isomeric fatty acyls much more challenging. Moreover, CID product ions that are diagnostic for AMPP derivatives of α-linolenic acid and γ-linolenic acid are present only in low abundance, consistent with previous reports [21].
Having successfully demonstrated this workflow for a relatively simple mixture of standards, the data-dependent OzID protocol was next applied to AMPP-FAs obtained from VC, a complex biological matrix containing multiple double bond positional isomers in the fatty acyl pool [29]. The GCMS trace of the FAME-derivatized sample is complex, with many chromatographic features not in the commercial standard (see Figure S4 in the Supporting Information). The spectrum obtained from a m/z 183 precursor ion scan of the AMPP-FA derivatives from a VC fraction is shown in Figure 3(a). This spectrum shows a pronounced series of peaks, ranging from C14–C24, with the most abundant ions being m/z 423 (AMPP-FA 16:0) and m/z 449 (AMPP-FA 18:1). Using the AMPP-specific precursor ion scan as a criterion to trigger ozonolysis in a data-dependent workflow, AMPP-functionalized FAs identified in the precursor ion scan were mass-selected and isolated in the presence of ozone for 2 s. The OzID spectra from each targeted unsaturated AMPP-FA derivative display diagnostic neutral losses, which indicate the position of the double bond(s) present. Three example OzID spectra of AMPP-FA 16:1 (m/z 421), AMPP-FA 17:1 (m/z 435), and AMPP-FA 18:1 (m/z 449) are shown in Figure 3(b), (c), and (d), respectively. These ions represent the most abundant unsaturated AMPP-FA species revealed by the precursor ion scan.
(a) Precursor ion scan (m/z 183) diagnostic of AMPP-FAs derived from VC. Peaks annotated in red indicate the most abundant monounsaturated species. OzID mass spectra of (b) AMPP-FA 16:1, (c) AMPP-FA 17:1, and (d) AMPP-FA 18:1, obtained from VC. The major double bond positions that are assigned based on the OzID product ions are indicated with the n-x nomenclature
OzID spectra of the monounsaturated compositions 16:1, 17:1, and 18:1 (shown in Figure 3) all contain multiple pairs of OzID product ions indicating that each fatty acyl sum composition is a mixture of at least 4 double bond positional isomers. For example, the OzID spectrum of AMPP-FA 16:1 shown in Figure 3(b) provides evidence for a dominant n-10 isomer (OzID product ions at m/z 297, 313), with minor contributions from n-12 (m/z 269), n-11 (m/z 283), n-8 (m/z 325), and n-7 (m/z 339). These data provide evidence for a mixture of isomers and are consistent with previous GC-MS studies that revealed multiple chromatographic features of the same elemental composition arising from hydrolyzed VC extracts (as FAMEs or picolinyl esters) [25, 27]. In these studies, at least 5 peaks were assigned to FA 16:1, 6 peaks to FA 17:1, and 5 to FA 18:1, but unambiguous assignment of all double bond positions was not possible [25]. Hauff and Vetter identified FA 17:1 (n-11) and FA 18:1 (n-10) as the predominant isomers of these fatty acyls in the total lipid pool of VC [27]. Although previous work identified FA 16:1 (n-7) as the major isomer of this sum composition in FAs from the global VC lipid pool, from the OzID data presented herein, we assign the major contributor in the TG fraction of VC as FA 16:1 (n-10), sapienic acid, which is known to be present in related human sebaceous secretions [39, 40]. It is important to note that the position of unsaturation relative to the charge has been shown to influence the rate of the ozonolysis reaction [23]. As such, quantification of the abundances of each double bond isomer would require careful calibration using fatty acid standards, some of which are not commercially available. A table listing all double bond locations identified by OzID from the derivatized TG fraction is provided in Supporting Information (Table S2). Note that ozonolysis provides no information regarding double bond stereochemistry nor chain branching. Prior studies identified both linear and branched-chain contributors to each sum composition assignment, and recent work has identified unique branching positions in FAs from VC beyond the conventional iso- and anteiso-methyl-branched FAs [41].
Compiling OzID spectral data from all precursor ions in a plot of precursor mass (x-axis) against the product ion masses (y-axis) produces a two-dimensional ion map, revealing the trends in positions of unsaturation with chain length, as shown in Figure 4. In this representation, precursor ions all fall along a diagonal line, marked in green. Oxidative cleavage of an AMPP-FA carbon–carbon double bond during OzID yields a pair of product ions at a predictable neutral loss from the precursor [37]. For a given double bond position relative to the AMPP-FA methyl terminus, (i.e., n-x), these product ions fall along a diagonal line parallel to the precursor ions, representing a fixed neutral offset. Integration along this line reveals a profile of individual double bond positions, similar to reconstructions of CID neutral losses recently reported in data-independent ion mobility MS experiments of human serum lipids [42].
A two-dimensional heat map representing unsaturation trends in AMPP-FA from VC. Each vertical line in the plot represents a single OzID spectrum, and raw peak intensities are proportional to dot size. Integration along the red lines yields a reconstructed neutral loss profile of all AMPP-FAs with a specific double bond position relative to the methyl terminus. Integration along the blue horizontal lines for a specific product ion m/z recreates an OzID precursor ion scan which identifies the double bond positions relative to the AMPP head group
Figure 5 shows reconstructed neutral loss scans for n-7, n-9, and n-10 double bond positions that are dominated by AMPP-FA 18:1 (m/z 449). Aside from this ion, n-7 double bonds are carried by AMPP-FA 16:1 (m/z 421, Figure 5(a)), whereas AMPP-FA 17:1 (m/z 435, Figure 5(b)) is a major carrier of n-9 double bonds. Interestingly, n-10 double bonds are present in both AMPP-FA 16:1 and AMPP-FA 18:1. AMPP-FA 20:1 (m/z 477) is also present in all three profiles.
Enzymatic activity in FA biosynthesis (e.g., elongation, desaturation, β-oxidation) is generally regulated by the distance from the carboxylate (i.e., Δy), rather than the methyl terminus [40]. Irrespective of chain length, all AMPP-FA carriers with a specific Δy double bond position will form the same OzID product ions, which fall along horizontal lines marked blue in Figure 4. Integrating along these lines reveals a profile specific to each Δy double bond position, as shown in Figure 6.
Of the five stearoyl-CoA desaturases (SCD) and three fatty acid desaturases (FADS) identified within mammals, only four enzymes (SCD1, SCD5, FADS1, and FADS2) are biologically active in humans [43,44,45,46]. Because of the enzyme-binding pocket properties and oxidizing metal sites, each of these desaturases catalyze dehydrogenation at a specific carbon distal to the carboxylate, leading to the formation of a double bond in a specific position; e.g., SCD1 and SCD5 act at the Δ9 carbon, FADS1 at Δ5, and FADS2 at the Δ6 carbon [40]. In combination with chain elongation also being effected from the carboxylate end of the fatty acid, this desaturation-site specificity means that the location of double bonds reveals both the enzyme reaction products and the elongation enzyme expression. Taking an example from the data presented here, the existence of the n-10 double bond within the VC TAG pool indicates the presence of FADS2Δ6 acting upon FA 16:0 to produce FA 16:1(n-10), with subsequent elongation yielding FA 18:1(n-10). Both of these monounsaturated fatty acids are present in the reconstructed n-10 neutral loss spectrum shown in Figure 5(c). Moreover, the OzID spectrum of AMPP-FA 20:1 (Figure 7(a)) shows neutral losses diagnostic for an n-10 double bond (m/z 353 and 369), possibly indicating that FA 18:1(n-10) can undergo further elongation to longer monounsaturated acyl chains. Also apparent in Figure 5(c) is an n-10 double bond in FA 18:2 (m/z 447), where the second double bond is located closer to the carboxylate, which is potential evidence for FA 18:1 (n-10) acting as a substrate for FADS1 Δ5 desaturation to form FA 18:2(n-10, n-13), commonly known as sebaleic acid and often identified within exogenous skin or hair oil secretions [47, 48]. Indeed, examination of the AMPP-FA 18:2 OzID spectrum (Figure 7(b)) shows evidence for multiple FA 18:2 isomers, including FA 18:2(n-10, n-13).
Conclusions
We have described a new implementation of ozone-induced dissociation to identify double bond position in fatty acids as AMPP derivatives on a triple quadrupole mass spectrometer. A precursor ion scan for a characteristic AMPP headgroup ion released upon CID (m/z 183) reveals the acyl chain length and degree of unsaturation, and subsequent OzID provides straightforward identification of the site(s) of unsaturation. Importantly, the coverage and dynamic range of this workflow is similar to conventional GCMS analysis of fatty acids as methyl ester derivatives.
Applying this OzID workflow to vernix caseosa, we were able to identify a range of double bond positional isomers that are not readily accessible by conventional analysis. By correlating the OzID product ion spectra to the mass-selected precursors in a two-dimensional manner, neutral loss scans can be reconstructed, yielding insight into the fatty acyl carriers for specific sites of unsaturation. The trend lines in such representations give an indication of the biosynthetic relationships between different unsaturated lipids and could be considered a snap shot of underlying lipid metabolism.
References
Eder, K.: Gas chromatographic analysis of fatty acid methyl esters. J. Chrom. B. 671, 113–131 (1995)
Christie, W.W., Han, X.: Lipid analysis: isolation, separation, identification and lipidomic analysis. Woodhead Publishing, Oxford (2010)
Deterding, L.J., Gross, M.L.: Fast-atom-bombardment and tandem mass spectrometry for determining structures of fatty acids as their picolinyl ester derivatives. Anal. Chim. Acta. 200, 431–445 (1987)
Deterding, L.J., Gross, M.L.: Tandem mass spectrometry for identifying fatty acid derivatives that undergo charge-remote fragmentations. Org. Mass Spectrom. 23, 169–177 (1988)
Dobson, G., Christie, W.W.: Structural analysis of fatty acids by mass spectrometry of picolinyl esters and dimethyloxazoline derivatives. TrAC Trends Anal. Chem. 15, 130–137 (1996)
Hancock, S.E., Poad, B.L.J., Batarseh, A., Abbott, S.K., Mitchell, T.W.: Advances and unresolved challenges in the structural characterization of isomeric lipids. Anal. Biochem. 524, 45–55 (2017)
Holčapek, M., Liebisch, G., Ekroos, K.: Lipidomic analysis. Anal. Chem. 90, 4249–4257 (2018)
Ryan, E., Reid, G.E.: Chemical derivatization and ultrahigh resolution and accurate mass spectrometry strategies for “shotgun” lipidome analysis. Acc. Chem. Res. 49, 1596–1604 (2016)
Murphy, R.C.: Tandem mass spectrometry of lipids: molecular analysis of complex lipids. Royal Society of Chemistry, Cambridge (2014)
Pittenauer, E., Allmaier, G.: The renaissance of high-energy CID for structural elucidation of complex lipids: MALDI-TOF/RTOF-MS of alkali cationized triacylglycerols. J Am Soc Mass Spectrom. 20, 1037–1047 (2009)
Ma, X., Chong, L., Tian, R., Shi, R., Hu, T.Y., Ouyang, Z., Xia, Y.: Identification and quantitation of lipid C=C location isomers: a shotgun lipidomics approach enabled by photochemical reaction. Proc. Natl. Acad. Sci. (USA). 113, 2573–2578 (2016)
Ma, X., Xia, Y.: Pinpointing double bonds in lipids by Paterno-Buchi reactions and mass spectrometry. Angew. Chem. (Int. Ed. Engl.). 53, 2592–2596 (2014)
Ma, X., Zhao, X., Li, J., Zhang, W., Cheng, J.-X., Ouyang, Z., Xia, Y.: Photochemical tagging for quantitation of unsaturated fatty acids by mass spectrometry. Anal. Chem. 88, 8931–8935 (2016)
Bollinger, J.G., Thompson, W., Lai, Y., Oslund, R.C., Hallstrand, T.S., Sadilek, M., Turecek, F., Gelb, M.H.: Improved sensitivity mass spectrometric detection of eicosanoids by charge reversal derivatization. Anal. Chem. 82, 6790–6796 (2010)
Yang, W.-C., Adamec, J., Regnier, F.E.: Enhancement of the LC/MS analysis of fatty acids through derivatization and stable isotope coding. Anal. Chem. 79, 5150–5157 (2007)
Wang, M., Han, R.H., Han, X.: Fatty acidomics: global analysis of lipid species containing a carboxyl group with a charge-remote fragmentation-assisted approach. Anal. Chem. 85, 9312–9320 (2013)
Bollinger, J.G., Rohan, G., Sadilek, M., Gelb, M.H.: LC/ESI-MS/MS detection of FAs by charge reversal derivatization with more than four orders of magnitude improvement in sensitivity. J. Lipid Res. 54, 3523–3530 (2013)
Frankfater, C., Jiang, X., Hsu, F.-F.: Characterization of long-chain fatty acid as N-(4-aminomethylphenyl) pyridinium derivative by MALDI LIFT-TOF/TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 29, 1688–1699 (2018)
Hancock, S.E., Ailuri, R., Marshall, D.L., Brown, S.H.J., Saville, J.T., Narreddula, V.R., Boase, N.R., Poad, B.L.J., Trevitt, A.J., Willcox, M.D.P., Kelso, M.J., Mitchell, T.W., Blanksby, S.J.: Mass spectrometry-directed structure elucidation and total synthesis of ultra-long chain (O-acyl)-ω-hydroxy fatty acids. J. Lipid Res. 59, 1510–1518 (2018)
Hsu, F.-F.: Characterization of hydroxyphthioceranoic and phthioceranoic acids by charge-switch derivatization and CID tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 27, 622–632 (2016)
Yang, K., Dilthey, B.G., Gross, R.W.: Identification and quantitation of fatty acid double bond positional isomers: a shotgun lipidomics approach using charge-switch derivatization. Anal. Chem. 85, 9742–9750 (2013)
Thomas, M.C., Mitchell, T.W., Harman, D.G., Deeley, J.M., Nealon, J.R., Blanksby, S.J.: Ozone-induced dissociation: elucidation of double bond position within mass-selected lipid ions. Anal. Chem. 80, 303–311 (2008)
Poad, B.L.J., Pham, H.T., Thomas, M.C., Nealon, J.R., Campbell, J.L., Mitchell, T.W., Blanksby, S.J.: Ozone-induced dissociation on a modified tandem linear ion-trap: observations of different reactivity for isomeric lipids. J. Am. Soc. Mass Spectrom. 21, 1989–1999 (2010)
Youssef, W., Wickett, R.R., Hoath, S.B.: Surface free energy characterization of vernix caseosa. Potential role in waterproofing the newborn infant. Skin Res. Technol. 7, 10–17 (2001)
Míková, R., Vrkoslav, V., Hanus, R., Háková, E., Hábová, Z., Doležal, A., Plavka, R., Coufal, P., Cvačka, J.: Newborn boys and girls differ in the lipid composition of vernix caseosa. PLOS ONE. 9, e99173 (2014)
Háková, E., Vrkoslav, V., Míková, R., Schwarzová-Pecková, K., Bosáková, Z., Cvačka, J.: Localization of double bonds in triacylglycerols using high-performance liquid chromatography/atmospheric pressure chemical ionization ion-trap mass spectrometry. Anal. Bioanal. Chem. 407, 5175–5188 (2015)
Hauff, S., Vetter, W.: Exploring the fatty acids of vernix caseosa in form of their methyl esters by off-line coupling of non-aqueous reversed phase high performance liquid chromatography and gas chromatography coupled to mass spectrometry. J. Chrom. A. 1217, 8270–8278 (2010)
Kalužíková, A., Vrkoslav, V., Harazim, E., Hoskovec, M., Plavka, R., Buděšínský, M., Bosáková, Z., Cvačka, J.: Cholesteryl esters of ω-(O-acyl)-hydroxy fatty acids in vernix caseosa. J. Lipid Res. 58, 1579–1590 (2017)
Ran-Ressler, R.R., Devapatla, S., Lawrence, P., Brenna, J.T.: Branched chain fatty acids are constituents of the normal healthy newborn gastrointestinal tract. Pediatr. Res. 64, 605 (2008)
Rissmann, R., Groenink, H.W.W., Weerheim, A.M., Hoath, S.B., Ponec, M., Bouwstra, J.A.: New insights into ultrastructure, lipid composition and organization of vernix caseosa. J. Invest. Dermatol. 126, 1823–1833 (2006)
Fahy, E., Subramaniam, S., Brown, H.A., Glass, C.K., Merrill, A.H., Murphy, R.C., Raetz, C.R.H., Russell, D.W., Seyama, Y., Shaw, W., Shimizu, T., Spener, F., van Meer, G., VanNieuwenhze, M.S., White, S.H., Witztum, J.L., Dennis, E.A.: A comprehensive classification system for lipids. J. Lipid Res. 46, 839–862 (2005)
Liebisch, G., Vizcaino, J.A., Kofeler, H., Trotzmuller, M., Griffiths, W.J., Schmitz, G., Spener, F., Wakelam, M.J.: Shorthand notation for lipid structures derived from mass spectrometry. J. Lipid Res. 54, 1523–1530 (2013)
The nomenclature of lipids (recommendations 1976). IUPAC-IUB Commission on Biochemical Nomenclature. J. Lipid Res. 19, 114–128 (1978)
Harazim, E., Vrkoslav, V., Buděšínský, M., Harazim, P., Svoboda, M., Plavka, R., Bosáková, Z., Cvačka, J.: Nonhydroxylated 1-O-acylceramides in vernix caseosa. J. Lipid Res. 59, 2164–2173 (2018)
Maccarone, A.T., Duldig, J., Mitchell, T.W., Blanksby, S.J., Duchoslav, E., Campbell, J.L.: Characterization of acyl chain position in unsaturated phosphatidylcholines using differential mobility-mass spectrometry. J. Lipid Res. 55, 1668–1677 (2014)
Poad, B.L.J., Maccarone, A.T., Yu, H., Mitchell, T.W., Saied, E.M., Arenz, C., Hornemann, T., Bull, J.N., Bieske, E.J., Blanksby, S.J.: Differential-mobility spectrometry of 1-deoxysphingosine isomers: new insights into the gas phase structures of ionized lipids. Anal. Chem. 90, 5343–5351 (2018)
Brown, S.H.J., Mitchell, T.W., Blanksby, S.J.: Analysis of unsaturated lipids by ozone-induced dissociation. Biochim. Biophys. Acta. 1811, 807–817 (2011)
Pham, H.T., Maccarone, A.T., Campbell, J.L., Mitchell, T.W., Blanksby, S.J.: Ozone-induced dissociation of conjugated lipids reveals significant reaction rate enhancements and characteristic odd-electron product ions. J. Am. Soc. Mass Spectrom. 24, 286–296 (2013)
Sansone, A., Melchiorre, M., Chatgilialoglu, C., Ferreri, C.: Hexadecenoic fatty acid isomers: a chemical biology approach for human plasma biomarker development. Chem. Res. Toxicol. 26, 1703–1709 (2013)
Guillou, H., Zadravec, D., Martin, P.G., Jacobsson, A.: The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog. Lipid Res. 49, 186–199 (2010)
Narreddula, V.R., Boase, N.R., Ailuri, R., Marshall, D.L., Poad, B.L.J., Kelso, M.J., Trevitt, A.J., Mitchell, T.W., Blanksby, S.J.: Introduction of a fixed-charge, photolabile derivative for enhanced structural elucidation of fatty acids. Anal. Chem. (2019). DOI: https://doi.org/10.1021/acs.analchem.9b01566
Hankin, J.A., Barkley, R.M., Zemski-Berry, K., Deng, Y., Murphy, R.C.: Mass spectrometric collisional activation and product ion mobility of human serum neutral lipid extracts. Anal. Chem. 88, 6274–6282 (2016)
Marquardt, A., Stöhr, H., White, K., Weber, B.H.F.: cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics. 66, 175–183 (2000)
Sprecher, H.: Biochemistry of essential fatty acids. Prog. Lipid Res. 20, 13–22 (1981)
Strittmatter, P., Spatz, L., Corcoran, D., Rogers, M.J., Setlow, B., Redline, R.: Purification and properties of rat liver microsomal stearyl coenzyme A desaturase. Proc. Natl. Acad. Sci. (USA). 71, 4565 (1974)
Wang, J., Yu, L., Schmidt, R.E., Su, C., Huang, X., Gould, K., Cao, G.: Characterization of HSCD5, a novel human stearoyl-CoA desaturase unique to primates. Biochem. Biophys. Res. Comm. 332, 735–742 (2005)
Destaillats, F., Guitard, M., Cruz-Hernandez, C.: Identification of Δ6-monounsaturated fatty acids in human hair and nail samples by gas-chromatography-mass-spectrometry using ionic-liquid coated capillary column. J. Chrom. A. 1218, 9384–9389 (2011)
Nicolaides, N.: Skin lipids: their biochemical uniqueness. Science. 186, 19 (1974)
Acknowledgements
This work was supported through funding from the Australian Research Council Discovery Program (DP150101715 and DP190101486). The results reported in this manuscript were obtained in the Central Analytical Research Facility (CARF), operated by the Institute for Future Environments (QUT). Access to CARF is supported by funding from the Science and Engineering Faculty (QUT).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
ESM 1
(DOCX 2594 kb)
Rights and permissions
About this article
Cite this article
Poad, B.L.J., Marshall, D.L., Harazim, E. et al. Combining Charge-Switch Derivatization with Ozone-Induced Dissociation for Fatty Acid Analysis. J. Am. Soc. Mass Spectrom. 30, 2135–2143 (2019). https://doi.org/10.1007/s13361-019-02285-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-019-02285-5