Abstract
Methodology for performing precursor and neutral loss scans in an RF scanning linear quadrupole ion trap is described and compared to the unconventional ac frequency scan technique. In the RF scanning variant, precursor ions are mass selectively excited by a fixed frequency resonance excitation signal at low Mathieu q while the RF amplitude is ramped linearly to pass ions through the point of excitation such that the excited ion’s m/z varies linearly with time. Ironically, a nonlinear ac frequency scan is still required for ejection of the product ions since their frequencies vary nonlinearly with the linearly varying RF amplitude. In the case of the precursor scan, the ejection frequency must be scanned so that it is fixed on a product ion m/z throughout the RF scan, whereas in the neutral loss scan, it must be scanned to maintain a constant mass offset from the excited precursor ions. Both simultaneous and sequential permutation scans are possible; only the former are demonstrated here. The scans described are performed on a variety of samples using different ionization sources: protonated amphetamine ions generated by nanoelectrospray ionization (nESI), explosives ionized by low-temperature plasma (LTP), and chemical warfare agent simulants sampled from a surface and analyzed with swab touch spray (TS). We lastly conclude that the ac frequency scan variant of these MS/MS scans is preferred due to electronic simplicity. In an accompanying manuscript, we thus describe the implementation of orthogonal double resonance precursor and neutral loss scans on the Mini 12 using constant RF voltage.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invented by Wolfgang Paul in the mid-twentieth century (though not used for mass analysis until much later) [1,2,3], the quadrupole ion trap (QIT) has become one of the most important tools for chemical analysis, largely due to high sensitivity but also because of selectivity and ease of applicability. Other key developments in the history of the QIT are the development of mass-selective instability [4], the first demonstration of collision-induced dissociation [5], the invention of linear ion traps with quadrupolar potentials in two dimensions instead of the QIT’s three [6,7,8], and the implementation of resonance ejection (both radial [9] and axial [8]) on linear ion traps. Dual linear ion traps (LITs) (e.g., Thermo Velos) [10], triple quadrupole linear ion traps (e.g., SCIEX QTRAP) [11, 12], and LIT-Orbitrap [13, 14] or LIT-TOF [15] have recently become commonplace in modern instrument suites. Another important development, though less recognized, was the first demonstration of the precursor ion scan in the QIT [16]. This method used simultaneously two resonance (low voltage ac) frequencies to activate precursor ions mass selectively as a function of time while also targeting a fixed product ion for ejection from the ion trap, all while keeping the main trapping RF voltage constant during the mass scan. Subsequently, data-dependent scans became the predominant method for precursor and neutral loss “scanning” in single 2D and 3D ion traps [17,18,19,20]. These are not single scans but are instead sets of scans from which precursor and neutral loss spectra are reconstructed.
Recent work has shown that data-independent precursor and neutral loss scans, usually accomplished with large, high-power, multi-analyzer instruments, are possible in single linear quadrupole ion traps as well by using orthogonal double resonance excitation [21,22,23]. These scans are methodologically similar to the 3D trap precursor scan implemented years ago, but precursor ions are excited and product ions are ejected orthogonally so as to prevent cross-talk between the two processes. The scans have further been developed into simultaneous and sequential MS/MS combinations and permutations in which multiple precursor scans are combined with neutral loss and product scans to maximize information gain from a single ion injection event [23]. To date, however, all data-independent precursor and neutral loss scans in a single QIT/LIT have involved scans of the low voltage ac frequency at constant RF voltage [24,25,26,27,28,29]. Under these conditions, the ions’ secular frequencies do not vary during the scan [30], making MS/MS scans easy to implement. Conventional ion traps, on the other hand, do not typically use ac frequency scanning for mass-selective operations but instead use highly precise linear ramps of the RF voltage (e.g., in mass-selective instability with resonance ejection) or multi-frequency waveforms [31,32,33] for ion manipulation.
Here, we demonstrate the corresponding precursor and neutral loss scans as well as some simultaneous combinations of precursor and neutral loss scans using an RF scanning linear quadrupole ion trap. We also compare this method with the ac frequency scan method developed previously. As we will see in the next section, the RF scan requires high precision not only in the RF voltage scan but also in the nonlinear ac frequency scan that targets product ions for ejection from the trap as the precursor ions fragment. We demonstrate these scans on a variety of samples using several different ambient ionization sources. The ambient ion sources combined with fully fleshed-out single analyzer MS/MS capabilities strengthen the capabilities of miniature mass spectrometers, for which these scans are particularly appropriate [34, 35].
Theory
We begin with the standard Mathieu q parameter for the linear ion trap while assuming conditions in which Mathieu a = 0 (i.e., no quadrupolar DC potential on the trap rods) [36,37,38],
where Vrf is the zero-to-peak amplitude of the main trapping sine wave (rf), m is the mass of the ion, r0 is the half distance between the rods, Ω is the angular RF frequency, z is the integer charge of the ion, and e is the elementary charge. We assume from this point on that the term “ze” collapses so that z is assumed to be the charge of the ion in coulombs. In the experiments demonstrated here, the RF amplitude is ramped linearly so that
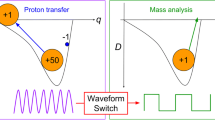
where dVrf/dt = constant and V0 is the RF voltage at the start of the mass scan. In the precursor ion scan, there are two waveforms (Fig. 1). First, a constant frequency sine wave at low Mathieu q is used for excitation of precursor ions where m/z is linearly related to time (because the RF voltage is scanned linearly). The product ion ejection frequency must be scanned so that it always ejects the selected product ion m/z. The product ion m/z is fixed and corresponds to a Mathieu parameter qpro, but because the RF voltage is scanned, the product ion’s Mathieu q parameter during the scan varies according to
meaning that the product ion ejection frequency for the precursor ion scan is simply varied so that a linear relationship between Mathieu q and time is maintained. The frequency scan rate depends on the RF voltage scan rate. We can then convert from Mathieu q to parameter β (see ref. [26]) and subsequently to ion secular frequency ω using the relationship β = 2ω/Ω, thereby giving the appropriate frequency sweep (Fig. 1, precursor ion scan, ACeject). This frequency scan must occur exactly in tandem with the RF scan so that resonance ejection of ions of similar m/z to the target ion does not occur. Therefore, high precision in both the RF voltage scan and ac frequency scan is required.
Scan table for precursor ion scans and neutral loss scans in an RF scanning linear quadrupole ion trap. The precursor ion excitation frequency is applied in the y dimension (where there is no detector) along with an artifact rejection frequency sweep (in the case of the neutral loss scan), whereas the product ion ejection frequency is placed on the x electrodes. Precursor ions fragment with a linear relationship between m/z and time and either a constant product ion m/z is ejected and detected or product ions with a shared mass offset from their respective precursor ions are detected
For the neutral loss scan, the product ion’s mass-to-charge ratio (m/z)pro changes as a function of time so as to maintain a constant mass offset, N, from the excited precursor ion m/z, (m/z)pre (which is excited at a frequency corresponding to Mathieu parameter qexc = constant),
so that
where (m/z)0 is the first precursor ion excited during the scan. Eq. 5 then simplifies to
which is a nonlinear frequency sweep (Fig. 1, neutral loss scan ACeject) that also must be calculated and loaded into an arbitrary waveform generator.
We note here that these equations were not used to calculate waveforms; they merely provide the theoretical foundation for the frequency scans. The method used in this work for calculating the frequency sweeps is described in the next section.
It is also worth briefly discussing the differences in implementation between the current work and previous work [21,22,23]. The current work we refer to as the “RF Scan” variant and the prior work we refer to as the “AC Scan” (or “‘Constant RF”) variant. In both cases, precursor ions are fragmented in the y dimension in the linear ion trap so that there is a linear relationship between fragmentation time and m/z. In the “RF Scan,” this is accomplished by linearly ramping the RF voltage while simultaneously applying a constant frequency auxiliary waveform in the y dimension. In the “AC Scan” case, fragmentation is accomplished by using an inverse Mathieu q scan [27] (a nonlinear frequency sweep with linear relationship between m/z and time) while the RF voltage is held constant. In the precursor scan, the product ions are then ejected with application in the x dimension (simultaneous with the excitation in y) of either (1) (“RF Scan”) the nonlinear frequency sweep described in Eq. 3, where the product ion Mathieu q is directly related to frequency, or (2) (“AC Scan”) a fixed frequency sine wave. For the neutral loss scan, the x dimension waveform is either (1) (“RF Scan”) the nonlinear frequency sweep described in Eq. 6, or (2) (“AC Scan”) a second inverse Mathieu q scan at constant mass (time) offset from the excitation frequency sweep. In the case of the neutral loss scan, an “Artifact Reject” frequency sweep must also be included to reject, into the y rods, precursor ions that do not fragment when they are excited. The “Artifact Reject” is generally identical to the product ion ejection frequency in terms of the curvature of frequency vs. time but is offset in frequency so as to be between the excitation and ejection frequencies.
Experimental
Chemicals
Amphetamine (m/z 136), methamphetamine (m/z 150), 3,4-methylenedioxyamphetamine (m/z 180), and 3,4-methylenedioxymethamphetamine (m/z 194) were purchased from Cerilliant (Round Rock, TX, USA) and diluted in methanol. These analytes were detected in the protonated form in the positive ion mode. HPLC grade methanol was purchased from Fisher Scientific (Hampton, NH, USA).
Explosives including 1,3,5-trinitrobenzene (m/z 213, M−), 2,6-dinitrotoluene (m/z 183, [M+H]−), 4-amino-2,6-dinitrotoluene (m/z 197, M−), and 2,4,6-trinitrotoluene (m/z 227, M−) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in methanol. These compounds were detected in the negative ion mode using low-temperature plasma ionization. Chemical warfare agent (CWA) hydrolysis products (i.e., simulants) ethyl methylphosphonate (m/z 123), isopropyl methylphosphonate-D7 (m/z 144), cyclohexyl methylphosphonate (m/z 177), and pinacolyl methylphosphonate (m/z 179), all m/z values for [M−H]−, were also purchased from Sigma and dissolved in methanol. CWAs were detected in the negative ion mode using swab touch spray.
Nanoelectrospray Ionization
Nanoelectrospray ionization [39] using a 1.5-kV potential was utilized for analysis of amphetamines. Borosilicate glass capillaries (1.5-mm O.D., 0.86-mm I.D.) from Sutter Instrument Co. (Novato, CA, USA) were pulled to 2-μm tip diameters using a Flaming/Brown micropipette puller (model P-97, Sutter Instrument Co.). The nanospray electrode holder (glass size 1.5 mm) was purchased from Warner Instruments (Hamden, CT, USA) and was fitted with 0.127-mm-diameter silver wire, part number 00303 (Alfa Aesar, Ward Hill, MA), as the electrode.
Low-temperature Plasma Ionization
The low-temperature plasma (LTP) probe and its use for the detection of explosives have been described elsewhere in detail [40, 41]. The probe consists of a glass tube (O.D. 5.95 mm, I.D. 3.88 mm) with an internal, stainless steel, grounded electrode of diameter 1.57 mm. A copper tape electrode surrounds the outside of the tube, and the glass serves as a dielectric barrier. Alternating current high voltage was applied by a PVM500 plasma driver purchased from Information Unlimited (Amherst, NH, USA) with voltage of 0.7–2.5 kV at a frequency of 21 kHz. Helium discharge gas was used at a flow rate of 0.4 L/min. Samples of 2 μL of explosives dissolved in methanol were placed on a glass slide, allowed to dry, and then moved to a custom-built holder mounted on a 3D moving stage. The glass slide was placed directly under the mass spectrometer inlet while the end of the LTP probe was 5 mm from the sample and inclined at an angle of 45°.
Swab Touch Spray Ionization
Swab touch spray experiments were performed using sterile rayon minitip swabs (model 160C) purchased from Copan Diagnostics (Murrieta, CA, USA). The swabs possess an aluminum handle and are mounted in a plastic cap that serves as a convenient holder. The swabs are commercialized for purposes other than ESI probes for MS analysis. However, they have been used without modification from their commercial form [42, 43]. Swab touch spray experiments were performed by soaking the rayon tip with methanol (approx. 40–50 μL) and then touching an area of a microscope glass slide that was previously spiked with a methanolic solution (which was let dry) containing ethyl methylphosphonate, isopropyl methylphosphonate-D7, cyclohexyl methylphosphonate, and pinacolyl methylphosphonate (500 ng of absolute material, each, deposited on the slide).
The swabs were placed vertically in front of the mass spectrometer using a custom ion source system. Electrospray was generated using methanol-ethanol 1:1 v/v as the solvent system. Electrospray was initiated after addition of solvent directly on the swab tip via a fused silica capillary and external syringe pump. The syringe pump flow rate was set at 50 μL/min for about 30 s, accounting for dead volume and wetting the swab tip. When the swab tip was visibly wet, high voltage (− 6.0 kV) was applied directly to the metallic handle. Solvent flow rate was changed to 20 μL/min. A red laser was used to visualize the spray plume.
Instrumentation
All experiments were performed using a Thermo LTQ linear ion trap (Thermo, San Jose, CA, USA) with RF frequency tuned to 1.1635 MHz (see ref. 6 for details of the instrument). The device has hyperbolic rod cross sections with y0 of 4.00 mm and x0 of 4.75 mm. Each rod is cut into three axial sections of 12-, 37-, and 12-mm length. The middle sections have a 0.25-mm slot cut 30 mm along the rod’s total length. The instrument was modified as described previously [22] to enable application of low voltage AC waveforms on both the x and y electrodes of the linear ion trap. All excitation and artifact rejection waveforms were applied to the y rods, and all ejection waveforms were applied to the x rods. Helium at an ion gauge reading of 0.60 x 10-5 Torr was used for collisional cooling and collision-induced dissociation.
For both neutral loss and precursor ion scan modes, a supplementary constant amplitude sine wave of fixed frequency (between 100 and 200 mVpp amplitude, usually between 130 kHz (q = 0.31) and 200 kHz (q = 0.46) was applied to the y electrodes to provide excitation for dissociation of precursor ions while the “Ultrazoom” scan function was used to linearly ramp the RF amplitude with high precision, thereby fragmenting precursor ions linearly with respect to time from low to high m/z. The “Ultrazoom” scan as commercially implemented ejects ions at a Mathieu q parameter of ~ 0.834 with a scan rate of 27 Da/s. We, however, replaced the auxiliary ac waveform with a first excitation waveform of constant frequency and amplitude and a second waveform of scanned frequency for product ion ejection. The product ion ejection waveform, described mathematically in the previous section, was a nonlinear frequency sweep with amplitude ~ 600 mVpp applied to the x electrodes at the same time as the RF ramp and constant frequency excitation signals. For neutral loss scans, a nonlinearly swept sine wave of constant amplitude and of lower frequency than the excitation waveform was used to reject leftover precursor ions to prevent them from being ejected by the product ion ejection frequency sweep. The supplementary waveforms were triggered at the start of the mass scan using the “scan out” trigger in the LTQ Tune Diagnostics menu. Automatic gain control was turned off for all experiments so that ion injection time was controlled manually and usually set at 10 ms.
Waveform Calculation
Product ion ejection waveforms were calculated in Matlab (Mathworks, Natick, MA) using an algorithm whose description follows. The commercial LTQ’s “Ultrazoom” scan was used to precisely control the RF amplitude, especially the linearity of the voltage ramp which ultimately determines the feasibility of these scan modes. Since the LTQ does not report the RF voltage to the user, the algorithm for calculating product ion ejection waveforms must be calibrated. It was experimentally determined that the Ultrazoom scan ejects ions at a frequency corresponding to Mathieu q = 0.834, and the scan rate at this q value is 27 Da/s. The start and end masses entered into the LTQ Tune software thus correspond to the m/z value at q = 0.834 at the start and end of the scan, respectively. The varied ejection m/z, linearly related to time due to the linearity of the RF ramp, and constant qeject were used to calculate the RF amplitude as a function of time through Eq. 1 (assuming a quadrupole coefficient of 1), with the RF frequency set at 1.1635 MHz and radius of x0 = 0.00475 m. This RF amplitude was then used to calculate the q value of the product ion as a function of time, and the q value was then converted to the ion’s secular frequency which was incorporated into an ac waveform using a phase accumulator as described previously [27]. The waveform (i.e., voltage vs. time) was exported from Matlab as a column vector (.csv) and imported into one of two Keysight 33612A function generators (Newark element14, SC, USA). The sampling rate for all waveforms was 10 MSa/s.
An important difference between the precursor ion scan and the neutral loss scan is that in the former the ejection frequency stays on a fixed m/z, whereas in the latter the product ion m/z is scanned to maintain a constant mass offset from the activated precursor ions. For the precursor ion scan, a constant m/z was converted to Mathieu q and then to secular frequency for each time point. For the neutral loss scan, the excited precursor ion m/z was calculated through Eq. 1 and the corresponding product ion m/z (m/zpre - N) was then converted to Mathieu q and hence secular frequency. For the neutral loss scan, it is important to include another frequency sweep on the y electrodes for artifact rejection because precursor ions leftover after the excitation event will otherwise be unintentionally ejected and detected via the product ion ejection frequency sweep [22]. For the RF ramp neutral loss scan, the product ion ejection waveform was also used for artifact rejection, except the sampling rate of the waveform generator was set lower (e.g., 7 MSa/s), thereby creating a waveform with similar curvature but lower frequencies than the product ion ejection waveform (green trace in Fig. 1). Thus, precursor ions surviving excitation were then neutralized on the y electrodes and therefore not detected.
Results and Discussion
Nanoelectrospray Ionization of Amphetamines
We first turn to a familiar system to acquire precursor and neutral loss spectra by scanning the RF voltage in a linear quadrupole ion trap: analysis of amphetamines ionized by nanoelectrospray ionization. Previously, we demonstrated precursor [21] and neutral loss [22] scans in an ac frequency scanning ion trap (at constant RF voltage) as well as simultaneous and sequential MS/MS combinations and permutations [23]. All of these scan modes can also be implemented while the RF amplitude is ramping linearly, though the implementation here is much more difficult because the ion secular frequencies vary nonlinearly as a function of the RF amplitude. For example, the four amphetamines shown in the full scan mass spectrum in Fig. 2a (amphetamine, methamphetamine, 3,4-methylenedioxyamphetamine, and 3,4-methylenedioxymethamphetamine) can be subdivided into different subsets based on (1) shared product ions (panels b–d) or (2) shared neutral losses (panels e–g). Amphetamine and methamphetamine are detected with a precursor ion scan of m/z 119, and 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine are detected with a precursor ion scan of m/z 163. All four compounds can be detected using a simultaneous double precursor ion scan (panel d) wherein the two product ion ejection frequencies used in panels b and c were summed and applied as a dipolar resonance ejection signal on the x electrodes of the linear ion trap while the precursor ions were excited at a single fixed frequency.
Precursor and neutral loss scans using an RF scanning linear ion trap. a Full scan mass spectrum of amphetamine, methamphetamine, 3,4-methylenedioxyamphetamine, and 3,4-methylenedioxymethamphetamine. b Precursor ion scan of m/z 119. c Precursor ion scan of m/z 163. d Simultaneous precursor ion scan of m/z 119 and 163. e Neutral loss scan of 17 Da. f Neutral loss scan of 31 Da. g Simultaneous neutral loss scan of 17 and 31 Da
Similarly, panel e shows a neutral loss scan of 17 Da to detect amphetamine and 3,4-methylenedioxyamphetamine, and a neutral loss scan of 31 Da detects the other two analytes. A simultaneous double neutral loss scan of 17 and 31 Da (panel g) again detects all the analytes in a single scan. In this case, the precursor ion excitation frequency and artifact rejection frequency sweep remain the same but the two product ion ejection frequencies (neutral loss of 17 Da and neutral loss of 31 Da) are summed and applied to the x electrodes simultaneously.
We now show that single ion trap precursor and neutral loss scans are useful for a wide variety of analytes ionized using several different ambient ionization sources. In particular, the ambient methods pair well with single analyzer MS/MS capabilities for applications in which miniature mass spectrometers are desired. As demonstrated here, these applications include low-temperature plasma ionization for detection of explosives on surfaces and swab touch spray ionization of chemical warfare agent simulants.
Low-temperature Plasma for Detection of Explosives on Surfaces
The target applications for single analyzer MS/MS scans are those which have significant resource constraints in terms of instrument size and power, access to sample, sample amount, and instrument portability. Detection of explosives is one application that can benefit from on-site instrumentation. Ambient plasma ionization methods (e.g., low-temperature plasma [41, 44, 45]) have been shown to be effective at detecting a wide array of alkyl and aryl nitrates and related compounds directly from surfaces with high sensitivity using both benchtop and portable instrumentation [40, 46]. A general survey scan that fits this class of compounds is a neutral loss of 30 Da, the neutral fragment corresponding to NO. Although not all aromatic nitrates fragment in this manner, many do so quite readily (see ref. [40]) and hence they can be simultaneously detected and confirmed by an MS/MS transition in a single mass scan segment. Figure 3a shows a full scan mass spectrum of four such analytes, namely 2,6-dinitrotoluene (m/z 183), 4-amino-2,6-dinitrotoluene (m/z 197), 1,3,5-trinitrobenzene (m/z 213), and 2,4,6-trinitrotoluene (m/z 227), ionized from a room temperature glass slide using low-temperature plasma ionization. A neutral loss scan of 30 Da is then shown in Fig. 3b. As little as 20 ng of each material was detected directly from the glass surface using the neutral loss scan. 2,6-Dinitrotoluene was not detected at 20 ng due to inefficient fragmentation but can be detected at higher concentrations.
Swab Touch Spray Ionization of Chemical Warfare Agent Simulants
Swab touch spray ionization [42, 43] is a recently developed variant of ambient touch spray ionization [47] wherein a rayon swab on an aluminum handle is used to collect material (e.g., oral fluid, solids from a surface [48], fibers in a forensic analysis) and subsequently solvent and high voltage are applied for ionization. In the experiment performed here, a mixture of chemical warfare agent simulants (500 ng each of ethyl methylphosphonate (m/z 123), isopropyl methylphosphonate-D7 (m/z 144), cyclohexyl methylphosphonate (m/z 177), and pinacolyl methylphosphonate (m/z 179), where all m/z values refer to [M−H]−) was deposited on a glass slide which was then allowed to dry, and then the slide was swabbed using a methanol-soaked rayon swab. The swab was then placed in a 3D–printed holder, 1:1 methanol/ethanol was continually supplied to the swab at a rate of ~ 20 uL/min, and − 6 kV was applied to generate ions. Figure 4a shows the full scan mass spectrum from this experiment; each of the CWA analytes is noted. A scan mode particularly suitable for these analytes is a precursor ion scan of m/z 95, which corresponds to the fragment ion CH3P=O(OH)O− (i.e., a methylphosphonate ion). The result from a precursor ion scan of m/z 95 is shown in Fig. 4b. All four CWAs are detected, though the signal-to-noise ratio is low due to inefficient fragmentation (He is used as collision gas on the LTQ). Note that isopropyl methylphosphonate-D7 fragments to m/z 96 but is also detected as a low abundance signal using the same precursor scan because of the closeness of the secular frequencies of the product ions. Further information about sensitivity and product ion selectivity can be found in the accompanying manuscript.
Precursor ion scan of chemical warfare agent simulants sampled from a glass substrate and ionized by swab touch spray. a Full scan mass spectrum of 500 ng each ethyl methylphosphonate (m/z 123), isopropyl methylphosphonic acid-D7 (m/z 144), cyclohexyl methylphosphonate (m/z 177), and pinacolyl methylphosphonate (m/z 179), all [M−H]−. b Precursor ion scan of m/z 95
One of the interesting features of this spectrum is the “humps” that are observed in the baseline. These may be caused by differences between the product ion’s actual secular frequency at a given point during the scan and the applied product ion ejection frequency, which can cause unintended resonance ejection as the applied ac frequency and product ion’s secular frequency drift away from each other. This characteristic makes calibration and optimization of the nonlinear frequency sweep quite difficult, especially in comparison to the ac frequency scan variant where the RF amplitude (and ion secular frequency) is constant [21]. When the RF is varied, the nonlinear ac frequency sweep must always line up, to a high degree of accuracy, with the highly precise RF voltage ramp so that the frequency sweep always targets the product ion of interest and not adjacent m/z ions. Other unwanted peaks can be observed when a precursor ion or product ion formed from fragmentation during the scan is ejected due to boundary instability (although this was not shown in any spectra here). Sometimes, these peaks can be distinguished from the precursor scan peaks by peak width; the former can be wider. In contrast, in the constant RF version of the precursor scan, the product ion ejection frequency must merely be tuned to the product ion’s fixed secular frequency, which is much easier to tune, and precursor ions cannot be ejected at the boundary because the rf does not vary during the scan.
Comparison of AC Frequency Scanning and RF Voltage Scanning for Ion Trap MS/MS
We now compare the two demonstrated variants of precursor and neutral loss scanning, paying particular attention to operational aspects of the scans. In previous experiments [21,22,23], the RF amplitude was kept constant during the scan and instead the inverse Mathieu q scan (nonlinear ac frequency scan with linear mass scale [27]) was used for mass-selective ion fragmentation while either a fixed frequency (precursor scan [21]) or inverse Mathieu q scan (neutral loss [22]) was used to eject a particular product ion while the precursor ions fragmented. In the current experiments, the RF amplitude is used more conventionally in that it is ramped linearly with time while a fixed frequency ac signal is used for precursor ion fragmentation. A nonlinearly scanned frequency is used for product ion ejection (Table 1). Conveniently, both MS/MS variants return data for which m/z is linearly related to time. In the ac frequency scan (constant rf) case, if the ac frequency is instead scanned linearly, then calibration is considerably more complicated [26]. In both the RF voltage scan case and ac frequency scan case, sequential and simultaneous scans are possible; however, for the RF scan, care must be taken not to eject the precursor ions at the Mathieu stability boundary (q = 0.908). In both scans, multigenerational capabilities [49, 50] are possible if the precursor ions are fragmented from high to low m/z, the direction opposite to that demonstrated in this paper. Unit resolution is observed in all of these scan modes for scan rates near 102 Da/s (the slowness of which is necessitated to obtain reasonable conversion of precursor ions to product ions). An advantage of the RF scan variant is identical fractional product ion mass range for all precursor ions since fragmentation is performed at constant frequency. Additionally, the collision energy can be ramped linearly with the RF amplitude without significantly altering mass calibration [51]. For the ac frequency scan case, the collision energy vs. time relationship would be considerably more complex.
Importantly, the ac frequency scan variant—at constant rf—is significantly simpler. For a precursor ion scan, a simple single frequency need be tuned for ejection of the product ion and a simple time offset between identical inverse Mathieu q scans is required for the neutral loss scan. There are also fewer artifact peaks because (1) the RF is not scanned and hence no ion’s secular frequency changes during the scan, and (2) the stable mass range in the ion trap does not vary with the scanning parameter, meaning fewer precursor ions and product ions generated during fragmentation will be ejected due to boundary instability. Hence, the ac frequency scan method ought to be the preferred MS/MS variant due to its simplicity and also because it generates more reliable data with fewer artifacts. A description of this mode of operation implemented on the Mini 12 mass spectrometer is described in the accompanying manuscript.
Conclusion
We have demonstrated an alternate scan mode in which precursor and neutral loss scans are possible in a single linear quadrupole ion trap using RF voltage scans combined with ac frequency scans. The scans are compatible with a wide range of ambient ionization sources and useful for a variety of purposes, including detection of explosives and chemical warfare agent simulants by low-temperature plasma ionization and touch spray ionization, respectively, and analysis of amphetamines by nanoelectrospray ionization. These cases and the corresponding ionization methods are merely illustrative, not comprehensive.
Compared with the ac frequency (constant rf) scan variant, the RF voltage scan version offers similar performance in terms of mass spectral resolution and fragmentation efficiency but can suffer from a greater proportion of artifact peaks due to boundary ejection and “unsyncing” of the RF voltage scan and product ion ejection frequency scan. In addition, the RF scan variant is significantly more complex, requiring simultaneous and highly precise scans of RF voltage and ac frequency. Nonetheless, because commercial mass spectrometers operate in the RF scan mode, this method may be more appropriate for applications where only current instrumentation is available.
References
Paul, W., Steinwedel, H.: A new mass spectrometer without a magnetic field. Z. Naturforsch. Sect. A. 8, 448–450 (1953)
Paul, W., Reinhard, H.P., von Zahn, U.: Das Elektrische Massenfilter Als Massenspektrometer Und Isotopentrenner. Z. Phys. 152, 143–182 (1958)
Paul, W.: Electromagnetic traps for charged and neutral particles. Rev. Mod. Phys. 62, 531 (1990)
Stafford, G.C., Kelley, P.E., Syka, J.E.P., Reynolds, W.E., Todd, J.F.J.: Recent improvements in and analytical applications of advanced ion trap technology. Int. J. Mass Spectrom. Ion Process. 60, 85–98 (1984)
Louris, J.N., Cooks, R.G., Syka, J.E.P., Kelley, P.E., Stafford, G.C., Todd, J.F.J.: Instrumentation, applications, and energy deposition in quadrupole ion-trap tandem mass-spectrometry. Anal. Chem. 59, 1677–1685 (1987)
Schwartz, J.C., Senko, M.W., Syka, J.E.: A two-dimensional quadrupole ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 13, 659–669 (2002)
Hager, J.W.: A new linear ion trap mass spectrometer. Rapid Commun. Mass Spectrom. 16, 512–526 (2002)
Londry, F.A., Hager, J.W.: Mass selective axial ion ejection from a linear quadrupole ion trap. J. Am. Soc. Mass Spectrom. 14, 1130–1147 (2003)
Fulford, J.E., Hoa, D.-N., Hughes, R.J., March, R.E., Bonner, R.F., Wong, G.J.: Radio-frequency mass selective excitation and resonant ejection of ions in a three-dimensional quadrupole ion trap. J. Vac. Sci. Technol. 17, 829–835 (1980)
Second, T.P., Blethrow, J.D., Schwartz, J.C., Merrihew, G.E., MacCoss, M.J., Swaney, D.L., Russell, J.D., Coon, J.J., Zabrouskov, V.: Dual-pressure linear ion trap mass spectrometer improving the analysis of complex protein mixtures. Anal. Chem. 81, 7757–7765 (2009)
Le Blanc, J.C., Hager, J.W., Ilisiu, A.M., Hunter, C., Zhong, F., Chu, I.: Unique scanning capabilities of a new hybrid linear ion trap mass spectrometer (Q TRAP) used for high sensitivity proteomics applications. Proteomics. 3, 859–869 (2003)
Hopfgartner, G., Varesio, E., Tschappat, V., Grivet, C., Bourgogne, E., Leuthold, L.A.: Triple quadrupole linear ion trap mass spectrometer for the analysis of small molecules and macromolecules. J. Mass Spectrom. 39, 845–855 (2004)
Olsen, J.V., Schwartz, J.C., Griep-Raming, J., Nielsen, M.L., Damoc, E., Denisov, E., Lange, O., Remes, P., Taylor, D., Splendore, M., Wouters, E.R., Senko, M., Makarov, A., Mann, M., Horning, S.: A dual pressure linear ion trap Orbitrap instrument with very high sequencing speed. Mol. Cell. Proteomics. 8, 2759–2769 (2009)
Makarov, A., Denisov, E., Kholomeev, A., Balschun, W., Lange, O., Strupat, K., Horning, S.: Performance evaluation of a hybrid linear ion trap/orbitrap mass spectrometer. Anal. Chem. 78, 2113–2120 (2006)
Collings, B.A., Campbell, J.M., Mao, D., Douglas, D.J.: A combined linear ion trap time-of-flight system with improved performance and MS(n) capabilities. Rapid Commun. Mass Spectrom. 15, 1777–1795 (2001)
Johnson, J.V., Pedder, R.E., Yost, R.A.: MS-MS parent scans on a quadrupole ion trap mass-spectrometer by simultaneous resonant excitation of multiple ions. Int. J. Mass Spectrom. Ion Process. 106, 197–212 (1991)
McClellan, J.E., Quarmby, S.T., Yost, R.A.: Parent and neutral loss monitoring on a quadrupole ion trap mass spectrometer: screening of acylcarnitines in complex mixtures. Anal. Chem. 74, 5799–5806 (2002)
Schwudke, D., Oegema, J., Burton, L., Entchev, E., Hannich, J.T., Ejsing, C.S., Kurzchalia, T., Shevchenko, A.: Lipid profiling by multiple precursor and neutral loss scanning driven by the data-dependent acquisition. Anal. Chem. 78, 585–595 (2006)
Swaney, D.L., McAlister, G.C., Coon, J.J.: Decision tree-driven tandem mass spectrometry for shotgun proteomics. Nat. Methods. 5, 959–964 (2008)
Schroeder, M.J., Shabanowitz, J., Schwartz, J.C., Hunt, D.F., Coon, J.J.: A neutral loss activation method for improved phosphopeptide sequence analysis by quadrupole ion trap mass spectrometry. Anal. Chem. 76, 3590–3598 (2004)
Snyder, D.T., Cooks, R.G.: Single analyzer precursor ion scans in a linear quadrupole ion trap using orthogonal double resonance excitation. J. Am. Soc. Mass Spectrom. 28, 1929–1938 (2017)
Snyder, D.T., Cooks, R.G.: Single analyzer neutral loss scans in a linear quadrupole ion trap using orthogonal double resonance excitation. Anal. Chem. 89, 8148–8155 (2017)
Snyder, D.T., Szalwinski, L., Cooks, R.G.: Simultaneous and sequential MS/MS combinations and permutations in a linear quadrupole ion trap. Anal. Chem. 89, 11053–11060 (2017)
Evans-Nguyen, T., Becker, L., Doroshenko, V., Cotter, R.J.: Development of a low power, high mass range mass spectrometer for Mars surface analysis. Int. J. Mass Spectrom. 278, 170–177 (2008)
Contreras, J.A., Murray, J.A., Tolley, S.E., Oliphant, J.L., Tolley, H.D., Lammert, S.A., Lee, E.D., Later, D.W., Lee, M.L.: Hand-portable gas chromatograph-toroidal ion trap mass spectrometer (GC-TMS) for detection of hazardous compounds. J. Am. Soc. Mass Spectrom. 19, 1425–1434 (2008)
Snyder, D.T., Pulliam, C.J., Cooks, R.G.: Calibration procedure for secular frequency scanning in an ion trap. Rapid Commun. Mass Spectrom. 30, 1190–1196 (2016)
Snyder, D.T., Pulliam, C.J., Cooks, R.G.: Linear mass scans in quadrupole ion traps using the inverse Mathieu q scan. Rapid Commun. Mass Spectrom. 30, 2369–2378 (2016)
Snyder, D.T., Pulliam, C.J., Wiley, J.S., Duncan, J., Cooks, R.G.: Experimental characterization of secular frequency scanning in ion trap mass spectrometers. J. Am. Soc. Mass Spectrom. 27, 1243–1255 (2016)
Snyder, D.T., Cooks, R.G.: Ion isolation and multigenerational collision-induced dissociation using the inverse Mathieu q scan. Rapid Commun. Mass Spectrom. 31, 200–206 (2017)
Douglas, D.J., Frank, A.J., Mao, D.: Linear ion traps in mass spectrometry. Mass Spectrom. Rev. 24, 1–29 (2005)
Julian, R.K., Cooks, R.G.: Broad-band excitation in the quadrupole ion-trap mass-spectrometer using shaped pulses created with the inverse fourier-transform. Anal. Chem. 65, 1827–1833 (1993)
Guan, S., Marshall, A.G.: Stored waveform inverse Fourier transform axial excitation/ejection for quadrupole ion trap mass spectrometry. Anal. Chem. 65, 1288–1294 (1993)
Guan, S., Marshall, A.G.: Stored waveform inverse Fourier transform (SWIFT) ion excitation in trapped-ion mass spectometry—theory and applications. Int. J. Mass Spectrom. Ion Process. 157/158, 5–37 (1996)
Ouyang, Z., Cooks, R.G.: Miniature mass spectrometers. Annu. Rev. Anal. Chem. 2, 187–214 (2009)
Snyder, D.T., Pulliam, C.J., Ouyang, Z., Cooks, R.G.: Miniature and fieldable mass spectrometers: recent advances. Anal. Chem. 88, 2–29 (2016)
March, R.E.: Quadrupole ion traps. Mass Spectrom. Rev. 28, 961–989 (2009)
March, R.E., Todd, J.F.J.: Practical aspects of trapped ion mass spectrometry, Vol. 4. CRC Press Taylor & Francis Group, Boca Raton (2010)
Dawson, P.H.: Quadrupole mass spectrometry and its applications. Elsevier, Amsterdam (2013)
Juraschek, R., Dulcks, T., Karas, M.: Nanoelectrospray—more than just a minimized-flow electrospray ionization source. J. Am. Soc. Mass Spectrom. 10, 300–308 (1999)
Garcia-Reyes, J.F., Harper, J.D., Salazar, G.A., Charipar, N.A., Ouyang, Z., Cooks, R.G.: Detection of explosives and related compounds by low-temperature plasma ambient ionization mass spectrometry. Anal. Chem. 83, 1084–1092 (2011)
Harper, J.D., Charipar, N.A., Mulligan, C.C., Zhang, X., Cooks, R.G., Ouyang, Z.: Low-temperature plasma probe for ambient desorption ionization. Anal. Chem. 80, 9097–9104 (2008)
Jarmusch, A.K., Pirro, V., Kerian, K.S., Cooks, R.G.: Detection of strep throat causing bacterium directly from medical swabs by touch spray-mass spectrometry. Analyst. 139, 4785–4789 (2014)
Pirro, V., Jarmusch, A.K., Vincenti, M., Cooks, R.G.: Direct drug analysis from oral fluid using medical swab touch spray mass spectrometry. Anal. Chim. Acta. 861, 47–54 (2015)
Hou, K., Xu, W., Xu, J., Cooks, R.G., Ouyang, Z.: Sampling wand for an ion trap mass spectrometer. Anal. Chem. 83, 1857–1861 (2011)
Wiley, J.S., Shelley, J.T., Cooks, R.G.: Handheld low-temperature plasma probe for portable “point-and-shoot” ambient ionization mass spectrometry. Anal. Chem. 85, 6545–6552 (2013)
Dalgleish, J.K., Hou, K., Ouyang, Z., Cooks, R.G.: In situ explosive detection using a miniature plasma ion source and a portable mass spectrometer. Anal. Lett. 45, 1440–1446 (2012)
Kerian, K.S., Jarmusch, A.K., Cooks, R.G.: Touch spray mass spectrometry for in situ analysis of complex samples. Analyst. 139, 2714–2720 (2014)
Fedick, P.W., Bain, R.M.: Swab touch spray mass spectrometry for rapid analysis of organic gunshot residue from human hand and various surfaces using commercial and fieldable mass spectrometry systems. Forensic Chem. 5, 53–57 (2017)
Snyder, D.T., Cooks, R.G.: Multigenerational broadband collision-induced dissociation of precursor ions in a linear ion trap. J. Am. Soc. Mass Spectrom. 27, 1914–1921 (2016)
Snyder, D.T., Fedick, P.W., Cooks, R.G.: Multigenerational collision-induced dissociation for characterization of organic compounds. Anal. Chem. 88, 9572–9581 (2016)
Doroshenko, V.M., Cotter, R.J.: Linear mass calibration in the quadrupole ion-trap mass-spectrometer. Rapid Commun. Mass Spectrom. 8, 766–776 (1994)
Acknowledgements
The authors thank Mark Carlsen (Purdue University Jonathan Amy Facility for Chemical Instrumentation) for LTQ modifications which made this work possible.
Funding
The authors acknowledge funding from NASA, Planetary Sciences Division, Science Mission Directorate (NNX16AJ25G). This work was also supported by a NASA Space Technology Research Fellowship (DTS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Snyder, D.T., Szalwinski, L.J., Schrader, R.L. et al. Precursor and Neutral Loss Scans in an RF Scanning Linear Quadrupole Ion Trap. J. Am. Soc. Mass Spectrom. 29, 1345–1354 (2018). https://doi.org/10.1007/s13361-018-1920-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-018-1920-3