Abstract
With the growth of the pharmaceutical industry, structural elucidation of drugs and derivatives using tandem mass spectrometry (MS2) has become essential for drug development and pharmacokinetics studies because of its high sensitivity and low sample requirement. Thus, research seeking to understand fundamental relationships between fragmentation patterns and precursor ion structures in the gas phase has gained attention. In this study, we investigate the fragmentation of the widely used anticancer drugs, doxorubicin (DOX), vinblastine (VBL), and vinorelbine (VRL), complexed by a singly charged proton or alkali metal ion (Li+, Na+, K+) in the gas phase. The drug–cation complexes exhibit distinct fragmentation patterns in tandem mass spectra as a function of cation size. The trends in fragmentation patterns are explicable in terms of structures derived from ion mobility mass spectrometry (IM-MS) and theoretical calculations.

ᅟ
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tandem mass spectrometry (MS2) has been applied to the structural characterization of various compounds, including peptides [1, 2], proteins [3, 4], polymers [5], lipids [6], and organic molecules [7]. Structures of unknown drug-related compounds [8–12] can also be deduced by comparing fragmentation patterns from MS2 with those of the precursor drugs. Structural elucidation of drugs and their derivatives using MS2, with its high sensitivity and low sample requirement, is emerging parallel to the growth of the pharmaceutical industry [13–15]. Research seeking fundamental insight into the relationship between fragmentation patterns and gas-phase precursor structure has gained attention in this area [16]. We previously reported an investigation of the fragmentation mechanism of paclitaxel and its metabolites based on structural characterization using collision-induced dissociation (CID) and ion mobility mass spectrometry (IM-MS) [17].
In this study, the above techniques revealed their utility for understanding the fragmentation of anticancer drugs. The widely used anticancer drugs, doxorubicin (DOX, Scheme 1a), vinblastine and vinorelbine (VBL and VRL, Scheme 1b) were investigated using low-energy CID. DOX is an anthracycline antitumor agent that intercalates into DNA and inhibits macromolecular biosynthesis [18]. VBL and VRL are vinca alkaloids, which act as inhibitors of microtubule polymerization [19, 20]. VBL and VRL have highly similar structures, but differ slightly in the catharanthine moiety (Scheme 1b). VRL has a nine-membered heterocycle and a hydroxyl group in the catharanthine unit, whereas VBL has an eight-membered ring without a hydroxyl group. Both classes of anticancer drugs have clinical activities against leukemia [21], breast, non-small lung, head, and neck cancers [22, 23].
Interactions of metal cations facilitate ionization of organic molecules devoid of basic and acidic functional groups via electrospray ionization (ESI) [24, 25]. In addition to enhancing ionization efficiency, metal cations can induce unique dissociation pathways of ions by specific interactions with functional groups followed by structural rearrangements [16, 26–30]. The size and binding properties of alkali metal ions produce a unique effect on their structural rearrangements and fragmentations patterns [28, 31–33]. The distinct fragmentation pathways and related gas-phase structures of anticancer drugs complexed with different alkali metal cations can enable multidimensional analysis of these species. IM-MS can be used to investigate fragmentation patterns in terms of precursor structures and has been applied to the structural characterization of small drug-like molecules [34–36], peptides, proteins, and polymers [37, 38]. In IM-MS, collisional interactions between ions and neutral buffer gas in an IM cell influence the traveling times of ions based on their charge, size, and shape. The collision cross-section (ΩD) of an ion can be determined from its traveling time [39–41]. Structural details of the ions can then be obtained by comparing the experimental ΩD (ΩD,exp) with the theoretical ΩD (ΩD,theo) from structural models generated by theoretical calculations.
Herein, we performed IM-MS experiments and theoretical calculations to correlate differences in the structures of cationized anticancer drugs with their fragmentation patterns from low-energy CID. The results show that DOX undergoes drastic changes in structure and fragmentation depending on the size of the metal cation. VRL and VBL yield specific protonated fragments irrespective of the adducted cation. IM-MS experiments and theoretical calculations were performed to understand the influence of gas-phase structural differences on fragmentation pathways.
Experimental
Materials
Doxorubicin hydrochloride (for DOX), vinblastine sulfate (for VBL), vinorelbine ditartrate (for VRL), and polyalanine were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. Lithium chloride and sodium chloride were purchased from Samchun Pure Chemical (Pyeongtaek, Korea). Potassium chloride was obtained from Junsei Chemical (Tokyo, Japan). HPLC grade water (J. T. Baker, Phillipsburg, NJ, USA) and formic acid (Sigma-Aldrich) were obtained commercially. The final concentration of analyte (DOX, VBL, or VRL) was adjusted to 5 μM. Protonated forms of the anticancer drugs were produced by adding 0.5% formic acid (%v/v) to the sample solution. Metal salts were added in 50-fold excess (molar) of each anticancer drug to obtain cationized drug ions. All sample solutions were prepared in water.
Collision-Induced Dissociation (CID)
Tandem mass spectra of cationized DOX, VBL, and VRL were obtained with a Thermo Scientific LTQ Velos dual ion trap mass spectrometer (Thermo Scientific, San Jose, CA, USA) in positive ion mode. A Thermo Scientific Q-Exactive hybrid quadrupole-Orbitrap mass spectrometer (Thermo Scientific) was used to assign fragments and to obtain accurate m/z values. Samples were injected into the ion trap mass spectrometer with a syringe pump at a flow rate of 10 μL/min; the electrospray voltage and capillary temperature were set to 4.0 kV and 200 °C, respectively. Thirty percent of the normalized collision energy was applied to all analyte ions. Samples were injected into the quadrupole-Orbitrap mass spectrometer with a syringe pump at a flow rate of 5 μL/min; the electrospray voltage and capillary temperature were 4.0 kV and 200 °C, respectively. Spectra of all samples were acquired over 200 scans and averaged. Molecular formulas were extracted from the peaks using Thermo Xcalibur 2.1 software (Thermo Scientific) for assignment of the fragment peaks.
Electrospray Ionization Traveling Wave Ion Mobility Mass Spectrometry (ESI-TWIM-MS)
IM-MS measurements were performed on a Waters Synapt G2 HDMS TWIM orthogonal acceleration time-of-flight mass spectrometer (Waters, Manchester, UK) in positive ion mode. The ESI parameters were: source temperature of 80 °C, capillary voltage of 3 kV, desolvation temperature of 150 °C, and cone voltage of 40 V. Helium cell flow rate was 180 mL/min. Nitrogen drift gas was introduced to the IM cell at a flow rate of 90 mL/min. The optimized traveling wave height and velocity were 40 V and 650 m/s, respectively; 120 spectra were obtained and averaged for each sample. The traveling times of the analyte ions were extracted using MassLynx software (ver. 4.1, Waters). Multiple peak fitting of IM spectra was performed with Origin 9.0 software (OriginLab, Northampton, MA, USA) with Gaussian functions. The R 2 values were greater than 0.99.
Collision Cross Section (ΩD)
The experimental collision cross sections (ΩD,exp) of cationized DOX, VBL, and VRL ions were obtained using a calibration method based on the previously reported ΩD value in helium drift gas [39]. Calibration curves were established by fitting the drift times of polyalanine ions with their published collision cross sections and plotting in a power relationship [42]. The results were corrected for mass-independent and mass-dependent times to obtain an effective drift time. The theoretical collision cross section (ΩD,theo) values of molecular models were calculated for cationized DOX, VBL, and VRL using the trajectory method implemented in MOBCAL [43]. The Lennard-Jones parameters of sodium ion were used for lithium and potassium ions, the parameters of which are absent in the original MOBCAL program [44]. To validate the use of sodium parameters, different Lennard-Jones parameters were tested, and they were shown to have negligible influences in the calculated ΩD,theo (Supplementary Figure S1). Calculation of experimental and theoretical N2 ΩD of protonated and sodiated drug complexes were carried out using polyalanine calibrant [45] and modified parameters [34, 46]. Then, theoretical N2 ΩD value of five energetically favored structures from each complex were averaged (Supplemental Table S4).
Molecular Modeling
The gas-phase structures and energetics of cationized DOX, VBL, and VRL were determined by a combination of molecular dynamics (MD) simulations using GROMACS 4.5.5 [47] and density functional theory (DFT) calculations using the Q-Chem 4.1 computational package and Q-Chem 3.2.0.3 version (Q-Chem Inc., Pittsburgh, PA, USA). The initial structures of DOX and VBL were obtained from the PDB database (4DX7 and 1Z2B, respectively) and VRL was obtained from the SuperLigands, a PDB ligand database. Values from the CHARMM General Force Field (CGENFF) were used to describe the potential of drug complexes. Pools of candidate models were first generated by simulated annealing using MD simulations. During the simulated annealing, simulation temperature was increased from 300 to 700 K for the first 100 ps, and then the increased temperature was maintained for the next 100 ps. Subsequently, the system was cooled to 300 K for 50 ps and the cooled temperature was maintained for 50 ps. Higher temperatures than 700 K were tested for validation of simulated annealing up to the point where the simulation becomes unstable and difference in cation binding sites is negligible. 1200 cycles for each DOX complex and 1800 cycles for each VBL and VRL complex were performed by repeating the aforementioned simulated annealing process. Candidate complexes were then clustered based on the cation binding sites in each drug molecule. Structures with the lowest energy in each cluster were subjected to geometry optimization using DFT and energies were calculated at the B3LYP/6-31G* level of theory. Structures with lowest energies and ΩD,theo that agrees with ΩD,exp within a standard deviation were selected as the possible structures. Zero-point energy corrections were applied to all candidate structures.
Results and Discussion
Fragmentation Patterns of Cationized DOX, VBL, and VRL
Singly charged DOX, VBL, and VRL ions containing proton or alkali metal cations (Li+, Na+, and K+) were generated by ESI (Supplementary Figure S2). No multimeric complexes were observed. Fragmentation pathways of the cationized anticancer drugs were investigated using low-energy CID. Figure 1 shows the MS2 spectra of DOX and the proposed structures for its CID fragments (see Supplementary Table S1 for detailed fragment assignment). The CID spectrum of [DOX + H]+ shows the dehydrated fragments [D2 – H2O + H]+, [D2 – 2H2O + H]+, and [D2 – 3H2O + H]+ from the aglycone fragment D2, which results from elimination of the amino sugar group (Scheme 1a). Elimination of the α-hydroxy ketone group from the aglycone fragment D2 produces [D1 + H]+. Bond cleavage in the sugar ring yields the protonated D3 fragment, which is confirmed by further CID of the daughter ions (MS3, Supplementary Figure S3). The MS2 fragments observed are in good agreement with previous studies, which reported that the major CID products of protonated DOX involve elimination of the amino sugar group [48–50].
Tandem mass spectra and proposed fragments of DOX. See Supplementary Table S1 for the relative abundance and experimental and theoretical m/z. Precursor ion of each cationized DOX complex is marked with arrows in tandem mass spectra
The dissociation patterns observed in the MS2 spectrum of [DOX + Li]+ are almost identical to those of [DOX + H]+. However, the relative abundance of the dehydrated forms of the aglycone fragment, [D2 – nH2O + Li]+, decreases while the relative abundance of the aglycone fragment [D2 + Li]+ increases. In addition, the D3 fragment is no longer observed in the CID of lithiated DOX. The MS2 spectrum of sodiated DOX ([DOX + Na]+) displays patterns that differ from those of protonated and lithiated DOX. The CID of [DOX + Na]+ yields the aglycone fragment [D2 + Na]+ and its mono-dehydrated form, [D2 – H2O + Na]+. The aglycone fragment [D2 + K]+ is the major CID product of potassiated DOX and the relative abundance of the dehydrated product, [D2 – H2O + K]+, decreases. New fragments D4 and D5 are observed, which result from internal dissociation of the sugar ring (see Supplementary Figure S3 for MS3 analysis). It is noteworthy that the relative abundance of the internal dehydrated fragments of DOX decreases with the increase in the size of charge-carrying cation (from proton to potassium), while the relative abundance of the intact D2 fragment increases. It is also notable that alkali metal ions are present in all fragments in the MS2 spectra of the metallated DOX, suggesting that these ions are attached to the aglycone moiety when the neutral amino sugar group or its internal fragments are eliminated.
Figure 2 displays the MS2 spectra and the proposed fragment structures of the vinca alkaloid drugs, VBL and VRL. Based on previous studies on their protonated forms [21, 51–53] and the m/z values obtained from ultrahigh resolution mass spectrometry (see Supplementary Tables S2 and S3 for VBL and VRL, respectively), the CID fragments from cationized VBL and VRL were analyzed. [VBL + H]+ shows four major fragments (Figure 2a), which are the catharanthine moiety fragments (VB1), fragments from internal dissociations of the vindoline moiety (VB2 and VB4), and fragments from elimination of an acetate or methyl ester group (60 Da, VB5). In addition, secondary fragments resulting from additional loss of water (H2O), methanol (CH3OH), and hydrogen atoms (2H) are observed in the spectrum. The CID spectra of alkali-metallated VBL show the VB5 fragments resulting from acetate or methyl ester group loss as the predominant species, whereas other fragments are detected in low abundances. Notably, the protonated fragment ions of VB5, VB4, VB2, and VB1, which were also observed in the case of protonated VBL, are observed in all CID spectra of metallated VBL. These fragments increase in abundance as the size of the charge-carrying cation increases.
Tandem mass spectra and proposed fragments of (a) VBL, and (b) VRL. See Supplementary Tables S2 and S3 for the relative abundance and experimental and theoretical m/z. Precursor ion of each cationized complex is marked with arrows in tandem mass spectra
The CID spectrum of [VRL + H]+ (Figure 2b) shows dissociation patterns similar to those of [VBL + H]+. Catharanthine moiety fragments (VR2), fragments from internal dissociation of the vindoline moiety (VR4 and VR6), and fragments from elimination of an acetate or methyl ester group (VR7) are observed in the MS2 spectra of [VRL + H]+ [54]. The vindoline moiety fragment (VR3) is also observed. The CID spectrum of [VRL + H]+ yields a distinct VR1 fragment, which results from internal dissociation of the eight-membered catharanthine ring in contrast to the CID of [VBL + H]+. The CID spectra of alkali-metallated VRL ions show more abundant VR7 fragments. Similar to metallated VBL, protonated fragment ions including VR7, VR6, and VR4 are also observed in all CID spectra of metallated VRL. It is notable that the abundance of protonated fragment ions resulting from internal dissociations of the vindoline moiety increases for VRL relative to VBL.
Ion Mobility Distributions and Structures of Cationized DOX, VBL, and VRL
The IM distributions of singly charged DOX, VBL, and VRL were examined to understand the distinct fragmentation patterns induced by cationization. Figure 3 shows the IM distributions of protonated and alkali-metallated DOX ions. [DOX + H]+ shows two conformations in its IM spectrum, one with ΩD,exp value of 153.1 Å2 and the other with 158.2 Å2. The DOX complexes with alkali metal cations adopt a single conformation with ΩD,exp values increasing uniformly from 158.2, 160.3, to 161.5 Å2 as the size of the metal ion increases from Li+, Na+, to K+, respectively.
The IM distributions of VBL and VRL were also examined to investigate the structural differences induced by cationization (Figure 4). Despite the complexity of the molecule, [VBL + H]+ exhibits a single conformation with ΩD,exp equal to 206.2 Å2. However, alkali-metallated VBL ions adopt two conformations. As the cation size increases, the ΩD,exp values of both conformer types increase gradually with the high-mobility conformer type becoming more favored than the low-mobility one. Cationized VRL ions exhibit similar behavior in that the larger conformer type is preferred over the smaller one as the size of the metal cation increases. However, protonated VRL exists in two conformations, one with a ΩD,exp of 193.9 Å2 and the other with 201.7 Å2. The IM distributions indicate that the low-mobility [VRL + H]+ conformer type is favored over the high-mobility one in the gas phase.
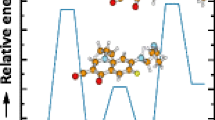
To understand the observed conformational changes of the anticancer drugs and their fragmentation patterns induced by attached cations, gas-phase structures of cationized DOX, VBL, and VRL were investigated by molecular modeling. Schemes 2, 3, and Supplementary Figure S4 show energetically favored structures of DOX, VBL, and VRL ions, for which the ΩD,theo values show agreement with ΩD,exp within a standard deviation. ΩD,theo and potential energies of representative structures are listed in Table 1. The ΩD,theo values for these structures in N2 drift gas agreed well with the ΩD,exp values calibrated for the N2 drift gas (Supplemental Table S4). In the high-mobility structure of [DOX + H]+ (Scheme 2a), the proton binds to the O16 atom of the carbonyl group and forms a hydrogen bond with the O17 atom in the aglycone moiety. This interaction induces the formation of a hydrogen bond between the O17 hydroxyl group and the O19 oxygen atom to hold the aglycone and amino sugar moieties together and generate a high-mobility structure. In the low-mobility structure (Scheme 2b), the proton interacts with the N9 atom and the O20 atom of the amino sugar moiety. No interaction with the aglycone moiety is observed.
Gas-phase structures of cationized DOX. Interactions between cations and polar functional group in DOX are indicated by dashed lines to the numbered atoms. (a) High-mobility and (b) low-mobility conformer type of [DOX + H]+. Proposed structures of (c) [DOX + Li]+, (d) [DOX + Na]+, and (e) [DOX + K]+. Only polar hydrogen atoms are shown in white for clarity
Gas-phase structures of cationized VBL. Interactions between cations and polar functional group in VBL are indicated by dashed lines to the numbered atoms. (a) Structures of [VBL + H]+. (b) Representation of the high-mobility conformer type of lithiated and sodiated VBL and (c) high-mobility conformer type of [VBL + K]+. (d) Low-mobility conformer type of [VBL + M]+ (M = Li, Na, K). Only polar hydrogen atoms are shown in white for clarity
Observation of a single IM distribution in alkali-metallated DOX (Figure 3) implies that DOX adopts a single conformation in the presence of Li+, Na+, and K+. The energetically favored structures, the ΩD,theo values of which are comparable to those of ΩD,exp, show a common structural feature in which the alkali metal cation is located in the groove between the amino sugar and the aglycone moieties (Scheme 2c, d, e). In [DOX + Li]+ and [DOX + Na]+, the metal cation interacts with O13 and O14 in the aglycone moiety and O18 and O19 in the amino sugar moiety. The potassium cation shows slightly different interactions, where K+ interacts with O12 and O14 in the aglycone moiety and the O20 hydroxyl group in the amino sugar moiety. The interaction with the hydroxyl group produces a slight twist in the amino sugar structure (bond angles of amino sugar moiety in Supplemental Table S5). However, the large size of the potassium ion compensates for the reduction in the size of DOX and results in a small increase in the overall ΩD,exp.
Scheme 3 shows representative structures of protonated and alkali-metallated VBL. The ΩD,exp of the single conformer type in the IM distribution of [VBL + H]+ agrees with ΩD,theo within a standard deviation and the energetically favored structure calculated (Scheme 3a). The proton in [VBL + H]+ binds to the N12 atom and interacts with the O22 atom via intramolecular hydrogen bonds in the vindoline moiety. Alkali-metallated VBL ions show two conformations in the gas phase (Figure 4a). The calculated VBL complex structures show similar coordination and interactions between VBL and the alkali metal ions regardless of size. In the high-mobility structures of [VBL + Li]+ and [VBL + Na]+, the metal cation interacts with the O17 in catharanthine moiety and the O24 in vindoline moiety to hold these two units close to one another (Scheme 3b). Besides O17 and O24, O21 also interacts with the potassium cation in the high-mobility structure of [VBL + K]+ (Scheme 3c). In contrast, the metal cation interacts exclusively with the vindoline moiety (O21, O22, O24) in the low-mobility structures of alkali-metallated VBL (Scheme 3d).
The IM distribution reveals the presence of two conformer types of protonated VRL (Figure 4b). The calculated structure indicates that VRL forms the high-mobility structure through hydrogen bonds with O17 in the catharanthine moiety and O24 in the vindoline moiety to hold the two units together (Supplementary Figure S4a). Owing to the smaller size of the catharanthine in VRL, the proton is captured by O17 and O24. The low-mobility form of [VRL + H]+ resembles the [VBL + H]+ structure, in which the proton interacts with the N12 atom and forms a hydrogen bond with the O22 atom (Supplementary Figure S4b). VRL shows coordination and alkali metal cation interactions that are similar to those of VBL (Supplementary Figure S4c, d, e), except for the high-mobility conformer type. In [VRL + Li]+ and [VRL + K]+, the high-mobility conformer types favor interactions with O17, O21, and O24, (Supplementary Figure S4c), whereas the high-mobility conformer type of [VRL + Na]+ favors interactions with O17 and O24. (Supplementary Figure S4d)
Binding energies (Table 2) indicate that the high-mobility conformer type of the vinca alkaloids becomes energetically more stable as the size of the metal cation increases, consistently with the IM distribution (Figure 4). For small alkali metal ions (Li+ and Na+), the metal cation tends to be localized at the vindoline moiety via strong ion–dipole interactions with O21, O22, and O24 (see Scheme 3d for VBL, and Supplementary Figure S4e for VRL). However, as the size of the metal cation increases to K+, the ion–dipole interactions with O21, O22, and O24 are reduced by steric hindrance to the dipoles. For strong ion–dipole interactions, the angle between the ion and the dipoles is observed to increase up to 180° to compensate the increase in the distance between K+ and the dipoles. However, the angles between K+ and dipole including O21 and O22 show relatively low value and negligible difference in correlation to cation size, due to steric hindrance on the dipoles (Supplemental Table S6). This induces less stable drug structure for capturing the cation. In the high-mobility structure, alkali metal ions interact with O17, O24, and O22, whereby the dipoles form a pseudo-plane with the metal ion at the center (Scheme 3b and c for VBL, Supplementary Figure S4c and d for VRL). Thus, it is concluded that K+ in potassiated vinca alkaloids interacts with O17, O24, and O22, which creates a stable interaction with the cation through relatively large angles and differences in the angle between the ion and the dipole (Supplemental Table S6).
Understanding the Fragmentation in Correlation with Gas-Phase Structure
We have investigated the structural characteristics of DOX, VBL, and VRL with proton and alkali metal cations and their distinct fragmentation patterns. For DOX, elimination of the amino sugar moiety becomes predominant as the size of the metal cation increases (Figure 1). In [DOX + Li]+ and [DOX + Na]+, dehydrated aglycone fragments arise via charge-induced fragmentation (CIF) [17], which results from strong interaction between the metal cation and hydroxyl groups (Scheme 4a). The binding energy of metal cation to DOX decreases as the metal ion size increases from Li+ to K+ (Table 2). In addition, the lengths of dissociable bonds, including C3–O18, C1–O14, and O13–C in [DOX + Li]+ are the greatest among the alkali metal complexes of DOX (Supplementary Table S7). This indicates that the stronger ion–dipole interactions of lithium ion with the hydroxyl groups (O13 and O14) in the aglycone moiety and oxygen atoms (O18 and O19) in the amino sugar moiety induce the elongation of the C–O bonds of the hydroxyl groups. Based on the previous study that fragmentation is correlated with bond length in gas-phase structure [55], the metalation-induced bond lengthening leads to more facile dehydration of the aglycone moiety via CID. Thus, lithiated DOX shows additional dehydrated fragments compared with sodiated DOX.
Proposed fragmentation mechanism for (a) DOX, (b) VBL, and VRL. Oxygen atoms interacting with metal ion are marked in red. Bond elongation and dissociation are indicated with full arrowed line and plain line, respectively. CIF and CRF stand for charge-induced fragmentation and charge-remote fragmentation, respectively
Elimination of the amino sugar moiety without additional dehydration predominates in the MS2 spectra of sodiated and potassiated DOX (Figure 1). It is concluded that additional dehydration of the aglycone fragment is prevented by the weak ion–dipole interactions between the metal cation and the hydroxyl groups in the aglycone unit. The distinct fragments resulting from internal dissociation of the amino sugar observed in the CID spectrum of [DOX + K]+ (Figure 1) are explained by charge-remote fragmentation (CRF) (Scheme 4a). CRF is a dissociation process that is independent of the charge state and charged site [56]. The weak binding of K+ to DOX localizes the charge of the potassium-bound oxygen atoms, but not enough to induce fragmentation of adjacent bonds. Thus, it prevents fragmentation of the aglycone moiety, also supported by the shorter bond lengths of aglycone moiety in Supplementary Table S7. However, the twisted structure of the amino sugar ring (Scheme 2e) that captures the large potassium ion induces internal dissociation and elimination of the amino sugar moiety (Figure 1), which is also confirmed by lengthened bonds of the amino sugar moiety (Supplementary Table S7).
For the vinca alkaloids, the alkali-metallated VBL and VRL ions show both metallated and protonated fragments regardless of metal cation attachment. As the metal cation size increases from Li+ to K+, the relative abundance of protonated fragments generally increases gradually, but dramatic increase is observed in the case of potassiated VBL (Figure 2 and Supplementary Figure S8). The increased abundance of the protonated fragments correlates with the predominance of the high-mobility conformer type, which increases as the size of metal cation increases (Figure 4a). In the high-mobility conformer type of alkali-metallated VBL, the optimized DFT structure (Scheme 3b and c) shows that a metal cation interacts with the catharanthine moiety (O17) and the vindoline moiety (O24 for Li+ and Na+, O21 and O24 for K+). Therefore, the enhanced abundance of protonated fragments in [VBL + K]+ is related to additional binding with the O21 atom. It is proposed that the protonated fragments of potassiated VBL are generated by CIF induced by the metal ion and its interaction with O21 and O24. Conversely, metal binding with O17 is the likely source of the metallated fragments, via CRF through loss of the methyl ester (which contains O21) or acetate group (which contains O24) (Scheme 4b). Consequently, [VBL + K]+ exhibits a greater abundance of protonated fragments than [VBL + Li]+ and [VBL + Na]+ due to higher number of coordination with vindoline moiety. It is also supported by the distances between alkali metal ion and bound oxygen atoms, which reveals that high-mobility structure of [VBL + K]+ has the largest differences between larger metal-O17 and smaller metal-O24, (Table 3).
Although the IM distributions of VRL are similar to those of VBL (Figure 4b), trends in the abundances of the protonated fragments in the tandem mass spectra of the metallated VRL ions differ from those of metallated VBL (Supplementary Figure S8). Owing to the additional binding with O21 (Supplementary Figure S4c), the protonated fragment abundance is greater in [VRL + Li]+ and [VRL + K]+ than in [VRL + Na]+ via CIF with the interaction of metal ion with vindoline moiety (Scheme 4b). The difference in the abundance of the protonated fragments from [VRL + Li]+ and [VRL + K]+ is also confirmed by the distance between metal ion and oxygen atoms. Metal-O17 binding become notably weaker in [VRL + K]+ than metal-O24, whereas [VRL + Li]+ shows the strongest binding between lithium and O17 (Table 3). Thus, potassiated VRL shows the greatest abundance of protonated fragments in Supplementary Figure S8. In addition, the abundance of protonated fragment ions from the dissociations at the vindoline moiety is increased with VRL than VBL (Supplementary Figure S8). The smaller ring size and additional double bond in the catharanthine moiety make the unit less flexible, which weakens the interactions of O17 with metal cations in the VRL. These weaker interactions lead to a higher abundance of protonated fragments in alkali-metallated VRL compared with VBL.
Conclusion
The complex structures and limited quantities of drugs and their derivatives make MS2 an essential tool for the structural characterization that is crucial to drug development and pharmacokinetics studies. Therefore, an understanding of the relationship between the precursor structure of ions and their fragmentation pathways will improve the quality of the related studies. In this paper, we suggest that structures of the alkali metal complexes of the major anticancer drugs, DOX, VBL, and VRL, derived from IM-MS and theoretical calculations have an important role in understanding the fragmentation patterns produced by CID. The coordination and binding strength of alkali metals to DOX promote distinct gas-phase structures and fragmentation patterns. VBL and VRL yield metallated and specific protonated fragments regardless of the identity of the adducted cation. The correlation between cation size and the increase in abundance of protonated fragments is explained by differences in coordination and molecular strain.
References
Lee, S.W., Kim, H.S., Beauchamp, J.L.: Salt bridge chemistry applied to gas-phase peptide sequencing: selective fragmentation of sodiated gas-phase peptide ions adjacent to aspartic acid residues. J. Am. Chem. Soc. 120, 3188–3195 (1998)
Wang, P., Polce, M.J., Bleiholder, C., Paizs, B., Wesdemiotis, C.: Structural characterization of peptides via tandem mass spectrometry of their dilithiated monocations. Int. J. Mass Spectrom. 249/250, 45–59 (2006)
Perdivara, I., Petrovich, R., Allinquant, B., Deterding, L.J., Tomer, K.B., Przybylski, M.: Elucidation of O-glycosylation structures of the beta-amyloid precursor protein by liquid chromatography-mass spectrometry using electron transfer dissociation and collision induced dissociation. J. Proteome Res. 8, 631–642 (2009)
Zhang, Z., Pan, H., Chen, X.: Mass spectrometry for structural characterization of therapeutic antibodies. Mass Spectrom. Rev. 28, 147–176 (2009)
Forsythe, J.G., Stow, S.M., Nefzger, H., Kwiecien, N.W., May, J.C., McLean, J.A., Hercules, D.M.: Structural characterization of methylenedianiline regioisomers by ion mobility-mass spectrometry, tandem mass spectrometry, and computational strategies: I. Electrospray spectra of 2-ring isomers. Anal. Chem. 86, 4362–4370 (2014)
El-Aneed, A., Banoub, J., Koen-Alonso, M., Boullanger, P., Lafont, D.: Establishment of mass spectrometric fingerprints of novel synthetic cholesteryl neoglycolipids: the presence of a unique C-glycoside species during electrospray ionization and during collision-induced dissociation tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 18, 294–310 (2007)
Kim, Y.H., Yoo, J.S., Lee, C.H., Goo, Y.M., Kim, M.S.: Application of fast atom bombardment combined with tandem mass spectrometry to the structural elucidation of O-demethylabierixin and related polyether antibiotics. J. Mass Spectrom. 31, 855–860 (1996)
Zhu, X., Kalyanaraman, N., Subramanian, R.: Enhanced screening of glutathione-trapped reactive metabolites by in-source collision-induced dissociation and extraction of product ion using UHPLC-high resolution mass spectrometry. Anal. Chem. 83, 9516–9523 (2011)
Levsen, K., Schiebel, H.M., Behnke, B., Dotzer, R., Dreher, W., Elend, M., Thiele, H.: Structure elucidation of phase II metabolites by tandem mass spectrometry: an overview. J. Chromatogr. A 1067, 55–72 (2005)
Nikolskiy, I., Siuzdak, G., Patti, G.J.: Discriminating precursors of common fragments for large-scale metabolite profiling by triple quadrupole mass spectrometry. Bioinformatics 31, 2017–2023 (2015)
Kasper, P.T., Rojas‐Chertó, M., Mistrik, R., Reijmers, T., Hankemeier, T., Vreeken, R.J.: Fragmentation trees for the structural characterisation of metabolites. Rapid Commun. Mass Spectrom. 26, 2275–2286 (2012)
Volk, K.J., Hill, S.E., Kerns, E.H., Lee, M.S.: Profiling degradants of paclitaxel using liquid chromatography mass spectrometry and liquid chromatography tandem mass spectrometry substructural techniques. J. Chromatogr. B Biomed. Sci. Appl. 696, 99–115 (1997)
Korfmacher, W.A.: Mass spectrometry for drug discovery and drug development. John Wiley & Sons, Inc., Hoboken, NJ (2013)
Guo, W.W., Qiu, F., Chen, X.Q., Ba, Y.Y., Wang, X., Wu, X.: In-vivo absorption of pinocembrin-7-O-beta-D-glucoside in rats and its in-vitro biotransformation. Sci. Rep. 6, 1–8 (2016)
Sadiq, M.W., Salehpour, M., Forsgard, N., Possnert, G., Hammarlund-Udenaes, M.: Morphine brain pharmacokinetics at very low concentrations studied with accelerator mass spectrometry and liquid chromatography-tandem mass spectrometry. Drug Metab. Dispos. 39, 174–179 (2011)
Duan, X., Luo, G., Chen, Y., Kong, X.: Effects of alkali metal ion cationization on fragmentation pathways of triazole-epothilone. J. Am. Soc. Mass Spectrom. 23, 1126–1134 (2012)
Lee, H.H., Hong, A., Cho, Y., Kim, S., Kim, W.J., Kim, H.I.: Structural characterization of anticancer drug paclitaxel and its metabolites using ion mobility mass spectrometry and tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 27, 329–338 (2016)
Sottani, C., Poggi, G., Melchiorre, F., Montagna, B., Minoia, C.: Simultaneous measurement of doxorubicin and reduced metabolite doxorubicinol by UHPLC-MS/MS in human plasma of HCC patients treated with TACE. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 915/916, 71–78 (2013)
Cao, X., Tai, Y., Sun, C., Wang, K., Pan, Y.: Characterization of impurities in semi-synthetic vinorelbine bitartrate by HPLC-MS with mass spectrometric shift technique. J. Pharm. Biomed. Anal. 39, 39–45 (2005)
Gigant, B., Wang, C., Ravelli, R.B., Roussi, F., Steinmetz, M.O., Curmi, P.A., Sobel, A., Knossow, M.: Structural basis for the regulation of tubulin by vinblastine. Nature 435, 519–522 (2005)
Favretto, D., Piovan, A., Cappelletti, E.M.: Electrospray ionization mass spectra and collision induced mass spectra of antitumour Catharanthus alkaloids. Rapid Commun. Mass Spectrom. 12, 982–984 (1998)
Damen, C.W.N., Lagas, J.S., Rosing, H., Schellens, J.H.M., Beijnen, J.H.: The bioanalysis of vinorelbine and 4‐O‐deacetylvinorelbine in human and mouse plasma using high‐performance liquid chromatography coupled with heated electrospray ionization tandem mass–spectrometry. Biomed. Chromatogr. 23, 1316–1325 (2009)
Ma, W., Wang, J., Guo, Q., Tu, P.: Simultaneous determination of doxorubicin and curcumin in rat plasma by LC-MS/MS and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 111, 215–221 (2015)
Kruve, A., Kaupmees, K., Liigand, J., Oss, M., Leito, I.: Sodium adduct formation efficiency in ESI source. J. Mass Spectrom. 48, 695–702 (2013)
Oss, M., Kruve, A., Herodes, K., Leito, I.: Electrospray ionization efficiency scale of organic compounds. Anal. Chem. 82, 2865–2872 (2010)
Sun, H., Wang, L., Pan, Y.: Gas-phase arylmethyl transfer and cyclodeamination of argentinated N-arylmethyl-pyridin-2-ylmethanimine. J. Am. Soc. Mass Spectrom. 25, 169–175 (2014)
Grossert, J.S., Cubero Herrera, L., Ramaley, L., Melanson, J.E.: Studying the chemistry of cationized triacylglycerols using electrospray ionization mass spectrometry and density functional theory computations. J. Am. Soc. Mass Spectrom. 25, 1421–1440 (2014)
Wei, J., Bristow, A.W., O'Connor, P.B.: The competitive influence of Li+, Na+, K+, Ag+, and H+ on the fragmentation of a PEGylated polymeric excipient. J. Am. Soc. Mass Spectrom. 26, 166–173 (2015)
Gaucher, S.P., Leary, J.A.: Influence of metal ion and coordination geometry on the gas phase dissociation and stereochemical differentiation of N-glycosides. Int. J. Mass Spectrom. 197, 139–148 (2000)
Morishetti, K.K., Russell, S.C., Zhao, X.N., Robinson, D.B., Ren, J.H.: Tandem mass spectrometry studies of protonated and alkali metalated peptoids: enhanced sequence coverage by metal cation addition. Int. J. Mass Spectrom. 308, 98–108 (2011)
Selby, T.L., Wesdemiotis, C., Lattimer, R.P.: Dissociation characteristics of [M + X]+ ions (X = H, Li, Na, K) from linear and cyclic polyglycols. J. Am. Soc. Mass Spectrom. 5, 1081–1092 (1994)
Schnier, P.D., Price, W.D., Strittmatter, E.F., Williams, E.R.: Dissociation energetics and mechanisms of leucine enkephalin (M + H)+ and (2M + X)+ ions (X = H, Li, Na, K, and Rb) measured by blackbody infrared radiative dissociation. J. Am. Soc. Mass Spectrom. 8, 771–780 (1997)
Fujihara, A., Sha, Y., Matsuo, S., Toyoda, M., Hayakawa, S.: High-energy collision-activated and electron-transfer dissociation of gas-phase complexes of tryptophan with Na+, K+, and Ca2+. Eur. Phys. J. D 68, 1–5 (2014)
Campuzano, I., Bush, M.F., Robinson, C.V., Beaumont, C., Richardson, K., Kim, H., Kim, H.I.: Structural characterization of drug-like compounds by ion mobility mass spectrometry: comparison of theoretical and experimentally derived nitrogen collision cross-sections. Anal. Chem. 84, 1026–1033 (2012)
O’Donnell, R.M., Sun, X., Harrington, P.B.: Pharmaceutical applications of ion mobility spectrometry. TrAC Trends Anal. Chem. 27, 44–53 (2008)
Reading, E., Munoz-Muriedas, J., Roberts, A.D., Dear, G.J., Robinson, C.V., Beaumont, C.: Elucidation of drug metabolite structural isomers using molecular modeling coupled with ion mobility mass spectrometry. Anal. Chem. 88, 2273–2280 (2016)
Lanucara, F., Holman, S.W., Gray, C.J., Eyers, C.E.: The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat. Chem. 6, 281–294 (2014)
Kim, K., Lee, J.W., Chang, T., Kim, H.I.: Characterization of polylactides with different stereoregularity using electrospray ionization ion mobility mass spectrometry. J. Am. Soc. Mass Spectrom. 25, 1771–1779 (2014)
Thalassinos, K., Grabenauer, M., Slade, S.E., Hilton, G.R., Bowers, M.T., Scrivens, J.H.: Characterization of phosphorylated peptides using traveling wave-based and drift cell ion mobility mass spectrometry. Anal. Chem. 81, 248–254 (2009)
Clemmer, D.E., Jarrold, M.F.: Ion mobility measurements and their applications to clusters and biomolecules. J. Mass Spectrom. 32, 577–592 (1997)
Wyttenbach, T., Kemper, P.R., Bowers, M.T.: Design of a new electrospray ion mobility mass spectrometer. Int. J. Mass Spectrom. 212, 13–23 (2001)
Valentine, S.J., Counterman, A.E., Clemmer, D.E.: A database of 660 peptide ion cross sections: use of intrinsic size parameters for bona fide predictions of cross sections. J. Am. Soc. Mass Spectrom. 10, 1188–1211 (1999)
Mesleh, M.F., Hunter, J.M., Shvartsburg, A.A., Schatz, G.C., Jarrold, M.F.: Structural information from ion mobility measurements: effects of the long-range potential. J. Phys. Chem. 100, 16082–16086 (1996)
Domalain, V., Tognetti, V., Hubert-Roux, M., Lange, C.M., Joubert, L., Baudoux, J., Rouden, J., Afonso, C.: Role of cationization and multimers formation for diastereomers differentiation by ion mobility-mass spectrometry. J. Am. Soc. Mass Spectrom. 24, 1437–1445 (2013)
Bush, M.F., Campuzano, I.D.G., Robinson, C.V.: Ion mobility mass spectrometry of peptide ions: effects of drift gas and calibration strategies. Anal. Chem. 84, 7124–7130 (2012)
Flick, T.G., Campuzano, I.D.G., Bartberger, M.D.: Structural resolution of 4-substituted proline diastereomers with ion mobility spectrometry via alkali metal ion cationization. Anal. Chem. 87, 3300–3307 (2015)
Hess, B., Kutzner, C., van der Spoel, D., Lindahl, E.: GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008)
Sottani, C., Tranfo, G., Bettinelli, M., Faranda, P., Spagnoli, M., Minoia, C.: Trace determination of anthracyclines in urine: a new high-performance liquid chromatography/tandem mass spectrometry method for assessing exposure of hospital personnel. Rapid Commun. Mass Spectrom. 18, 2426–2436 (2004)
Sleno, L., Campagna-Slater, V., Volmer, D.A.: Dissociation reactions of protonated anthracycline antibiotics following electrospray ionization-tandem mass spectrometry. Int. J. Mass Spectrom. 255/256, 130–138 (2006)
Sardi, I., la Marca, G., Giovannini, M.G., Malvagia, S., Guerrini, R., Genitori, L., Massimino, M., Arico, M.: Detection of doxorubicin hydrochloride accumulation in the rat brain after morphine treatment by mass spectrometry. Cancer Chemother. Pharmacol. 67, 1333–1340 (2011)
Trim, P.J., Henson, C.M., Avery, J.L., McEwen, A., Snel, M.F., Claude, E., Marshall, P.S., West, A., Princivalle, A.P., Clench, M.R.: Matrix-assisted laser desorption/ionization-ion mobility separation-mass spectrometry imaging of vinblastine in whole body tissue sections. Anal. Chem. 80, 8628–8634 (2008)
Dubrovay, Z., Háda, V., Béni, Z., Szántay, C.: NMR and mass spectrometric characterization of vinblastine, vincristine, and some new related impurities–Part I. J. Pharm. Biomed. Anal. 84, 293–308 (2013)
Béni, Z., Háda, V., Dubrovay, Z., Szántay, C.: Structure elucidation of indole–indoline type alkaloids: a retrospective account from the point of view of current NMR and MS technology. J. Pharm. Biomed. Anal. 69, 106–124 (2012)
De Graeve, J., Van Heugen, J.C., Zorza, G., Fahy, J., Puozzo, C.: Metabolism pathway of vinorelbine (Navelbine) in human: characterization of the metabolites by HPLC-MS/MS. J. Pharm. Biomed. Anal. 47, 47–58 (2008)
Wright, P., Alex, A., Harvey, S., Parsons, T., Pullen, F.: Understanding collision-induced dissociation of dofetilide: a case study in the application of density functional theory as an aid to mass spectral interpretation. Analyst 138, 6869–6880 (2013)
Gross, M.L.: Charge-remote fragmentation: an account of research on mechanisms and applications. Int. J. Mass Spectrom. 200, 611–624 (2000)
Acknowledgments
This work was supported by a Basic Research Program (no. 2016R1A2B4013089) through the National Research Foundation (NRF) of Korea funded by the Ministry of Science, ICT, and Future Planning (MSIP), a Basic Science Research Program (no. 20100020209) through the NRF of Korea funded by the Ministry of Education, and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health and Welfare of Korea (grant no. HT13C-0011-040013). We would like to acknowledge the financial support from the R&D Convergence Program of NST (National Research Council of Science and Technology) of Republic of Korea. This work was also supported by the National Institute of Supercomputing and Network/Korea Institute of Science and Technology Information, with supercomputing resources including technical support (KSC-2016-S1-0003).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2.01 mb)
Rights and permissions
About this article
Cite this article
Hong, A., Lee, H.H., Heo, C.E. et al. Distinct Fragmentation Pathways of Anticancer Drugs Induced by Charge-Carrying Cations in the Gas Phase. J. Am. Soc. Mass Spectrom. 28, 628–637 (2017). https://doi.org/10.1007/s13361-016-1559-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-016-1559-x