Abstract
The fragment ions observed with time-of-flight (TOF) and quadrupole ion trap (QIT) TOF mass spectrometers (MS) combined with matrix-assisted laser desorption/ionization in-source decay (MALDI-ISD) experiments of phosphorylated analytes β-casein and its model peptide were compared from the standpoint of the residence timeframe of analyte and fragment ions in the MALDI ion source and QIT cell. The QIT-TOF MS gave fragment c-, z′-, z-ANL, y-, and b-ions, and further degraded fragments originating from the loss of neutrals such as H2O, NH3, CH2O (from serine), C2H4O (from threonine), and H3PO4, whereas the TOF MS merely showed MALDI source-generated fragment c-, z′-, z-ANL, y-, and w-ions. The fragment ions observed in the QIT-TOF MS could be explained by the injection of the source-generated ions into the QIT cell or a cooperative effect of a little internal energy deposition, a long residence timeframe (140 ms) in the QIT cell, and specific amino acid effects on low-energy CID, whereas the source-generated fragments (c-, z′-, z-ANL, y-, and w-ions) could be a result of prompt radical-initiated fragmentation of hydrogen-abundant radical ions [M + H + H]+ and [M + H – H]– within the 53 ns timeframe, which corresponds to the delayed extraction time. The further degraded fragment b/y-ions produced in the QIT cell were confirmed by positive- and negative-ion low-energy CID experiments performed on the source-generated ions (c-, z′-, and y-ions). The loss of phosphoric acid (98 u) from analyte and fragment ions can be explained by a slow ergodic fragmentation independent of positive and negative charges.

ᅟ
Similar content being viewed by others
Introduction
Mass spectrometry (MS) combined with soft-ionization methods such as matrix-assisted laser desorption/ionization (MALDI) [1, 2] and electrospray ionization (ESI) [3, 4] is an indispensable tool for analyzing biological compounds such as peptide and protein. MALDI MS is often used for analyzing phosphorylated proteins by combining with enzymatic digestion and tandem mass spectrometry (MS/MS or MSn) [5, 6]. A method of in-source decay (ISD) coupled with MALDI has also been employed for analyzing intact proteins [7, 8]. The resulting and detectable fragment ions in MS/MS and MALDI-ISD experiments are dependent upon the ion optics such as quadrupole ion trap (QIT), triple-stage quadrupole (TSQ), tandem time-of-flight (TOF/TOF), and Fourier transform ion cyclotron resonance (FTICR) types [6–8]. Furthermore, the peptide product ions a, b, c, d, x, y, z, and w observable in high-energy collision-induced dissociation (CID) (Scheme 1) [9] and b- and y-ions in low-energy CID, are influenced by several factors such as internal energy E deposited in precursor ions, activation methods, and the ion residence timeframe in the reaction regions, which include the ion source and collision cell. It is expected that in general the fragment ions observed in CID and mass spectra correspond to the product ions generated within the residence timeframe in the collision cells of QIT (ca. 100 ms), TSQ (ca. 10 μs), TOF/TOF (ca. 1 μs) and FTICR (ca. 1 s), while the fragment ions observed in MALDI-ISD spectra using TOF MS are generated within the delay time (typically 100 ns) in the ion source.
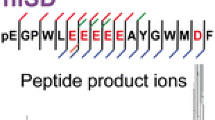
Nomenclature for peptide fragments [9]
The fragment ions that can be observed in CID and mass spectra are nearly always governed by the classic rate constant k (E) for fragmentation reactions as follows [10]:
where υ represents the frequency factor of the bond oscillators in a molecule, E and E 0 represent the internal energy and the threshold energy for cleavage of the bond of interest, and s represents the number of internal degrees of freedom. The frequency factor υ lies in the range of 1013 to 1014 s–1 [10], so that the timeframe for one oscillation is around several tens of femtoseconds (10–14 s). In the case of mass spectrometry using ESI, MALDI, and CID methods, the timeframes described above are longer than that of the bond oscillation of the analytes. It is also of importance to recognize further that bond cleavage of gaseous ions in mass spectrometry can be initiated by radical or charge sites of analyte ions such as M+•, [M + nH]n+, and [M – nH]n–. The radical species containing unpaired electrons are generally unstable and have a lower threshold energy E 0 of fragmentation, so that the open shell radical species initiate prompt cleavages. It is well known that radical sites (unpaired electron) in particular of the organic radical cations M+• generated in electron ionization (EI) ion sources are extremely active and promptly change the molecular ion structure due to intramolecular migration of hydrogen or alkyl chains [11–13]. In this case, the structural change in the M+- ions occurs in the ion source within ca. 100 ns. Furthermore, the radical-initiated fragmentation (RIF) rapidly occurs immediately after the formation of the radical cations M+• in the EI ion source or the hydrogen-abundant radical molecules [M + H]-generated in the MALDI ion source (Scheme 2) [14]. In contrast, the protonated and deprotonated analytes [M + nH]n+ and [M – nH]n– lacking radical sites are relatively stable because of their closed-shell structure. In the case of the closed-shell ions such as [M + nH]n+ and [M – nH]n–, the fragment ions can be obtained with CID methods via low-energy charge-initiated fragmentation (CIF) or high-energy charge-remote fragmentation (CRF). It should be noted that in contrast, the MALDI-ISD occurs essentially attributable to RIF processes independent of the presence of positive and negative charges [8, 14]. Although the ISD fragments such as c- and z′-ions generated within μs in the MALDI ion source can be detected with linear or reflectron TOF MS within the μs timeframe, the source-generated fragment ions from QIT-TOF MS can be detected after ca. 100 ms through collisional cooling with cooling gas in the ion trap cell. In the case of QIT-TOF MS, the MALDI source-generated fragments such as c/z′-ions degrade by energy randomization through frequent oscillations of each bond of the fragment ions within the relatively long residence timeframe of 100 ms in the QIT cell. Therefore, the MALDI-QIT-TOF mass spectra can give the fragment ions originating from the degradation of the source-generated c/z′-ions and surviving c/z′-ions, whereas MALDI-TOF mass spectra merely show the source-generated fragment ions.
Nomenclature for MALDI-ISD fragments [14]
Here we compare and analyze the fragment ions detected using two mass spectrometers with different ion optics, namely TOF MS and QIT-TOF MS coupled with positive- and negative-ion MALDI-ISD experiments of a phosphorylated protein β-casein and its model peptide. The β-casein and model peptide contain 209 and 29 amino acids, respectively, and have five and four phosphate groups. These analytes are characterized by the loss of phosphoric acid (H3PO4, 98 u) from both [M + H]+ and [M – H]– ions. The origin of the fragment ions detected by QIT-TOF MS can be classified into three type of reactions: (1) the radical-initiated fragmentation (RIF) of hydrogen-abundant radical ions [M + H + H]+• and [M + H – H]–•, (2) the charge-initiated fragmentation (CIF) of the source-generated fragment c-, z′-, and y-ions, and (3) the charge-remote fragmentation (CRF) of the source-generated ions in the QIT cell. It can be concluded that QIT-TOF MS detects the fragment ions originating from RIF, CIF and slow CRF reactions in both the QIT cell and the MALDI ion source, whereas TOF MS exclusively detects the fragment ions originating from prompt RIF reactions in the MALDI ion source.
Experimental
Reagents
5-Amino-1-naphthol (5,1-ANL) as a matrix material was purchased from Tokyo Chemical Industry (Tokyo, Japan). The phosphorylated protein β-casein and its phosphorylated model peptide were purchased from Sigma Aldrich (Steinheim, Germany) and Peptide Institute (Minoh, Osaka, Japan), respectively. Acetonitrile was purchased from Wako Pure Chemicals (Osaka, Japan). Water used in all experiments was purified using a MilliQ water purification system from Millipore (Billerica, MA, USA). All reagents were used without further purification.
Mass Spectrometry
MALDI-ISD mass spectra were acquired on a linear time-of-flight (MALDI-TOF) mass spectrometer AXIMA Performance (Shimadzu/Kratos, Manchester, UK) equipped with a nitrogen laser (337 nm wavelength, 4 ns pulse width) operating at a pulse rate of 50 Hz. The laser spot size on the target substrate was ca. 100 μm in diameter. The ions generated in the ion source of the MALDI were accelerated using 20 kV with a delayed extraction time of 53 ns. The analyzer was operated in linear mode and the ions were detected using a secondary electron multiplier. A total of 500 shots were accumulated for each mass spectrum acquisition. MALDI-ISD and subsequent MALDI-ISD/CID spectra were obtained using a quadrupole ion trap time-of-flight (QIT-TOF) mass spectrometer AXIMA Resonance (Shimadzu/Kratos) equipped with a nitrogen laser (337 nm wavelength, 3 ns pulse width) operating at a pulse rate of 10 Hz. The ISD fragment ions injected into the QIT cell were decelerated by a static electric field of 50 V applied to the far end-cap and collided with the pulsed helium gas for 140 ms for cooling and argon gas for 30 ms for the CID experiment. The product ions generated in the QIT cell were extracted by applying a potential between the two end caps and pulsed into the TOF system with an accelerating voltage of 10 kV.
Sample Preparation
Analytes were dissolved in water at a concentration of 20 pmol/μL. The matrix 5,1-ANL was dissolved in a solution of water/acetonitrile (3:7, v/v). The sample solution was prepared by mixing a volume of 10 μL of analyte solution with a volume of 10 μL of matrix solution. The molar ratio of analyte and matrix molecules was ca. 1:1000. A volume of 0.5 μL of the sample solution was deposited onto a stainless-steel MALDI plate and the solvents were removed by allowing evaporation in air at room temperature.
Results and Discussion
ISD Spectra of β-Casein and Its Model Peptide Using MALDI-TOF and MALDI-QIT-TOF MS
The MALDI-ISD spectra of a phosphorylated model peptide obtained using MALDI-TOF MS and MALDI-QIT-TOF MS are shown in Figures 1 and 2, respectively. Both positive- and negative-ion ISD spectra with TOF MS (Figure 1) showed just c-ions and minor peaks corresponding to z′-, z-ANL, y-, and w-ions (Scheme 2), whereas the ISD spectra from QIT-TOF MS showed b-, c-, y-, and z′-ions (Table 1 and Figure 2). Figure 2 shows further characteristic fragment ions originating from the loss of phosphoric acid (H3PO4, 98 u) from b-, c-, y-ions, and analyte ions [M + H]+ and [M – H]– (Table 1), while Figure 1 does not show any ion peaks originating from the loss of phosphoric acid. The fragment ions observed are summarized in Table 1.
The ISD spectra of β-casein obtained with TOF MS and QIT-TOF MS are shown in Figures 3 and 4, respectively. Both positive- and negative-ion ISD spectra from TOF MS show only c-, y-, and z′-ions (Figure 3), whereas the ISD spectra from QIT-TOF MS show b-, c-, y-, and z′-fragment ions (Figure 4). Furthermore, the QIT-TOF mass spectra show fragment ions originating from the loss of phosphoric acid (H3PO4, 98 u) from c-ions, whereas the TOF mass spectra did not show any peaks originating from the loss of phosphoric acid. The observed fragment ions are summarized in Table 2.
Classifying Fragment Ions Generated in MALDI Ion Source and Ion Trap Cell
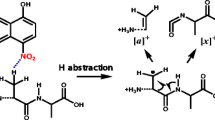
It is of importance to classify the ion peaks observed in ISD spectra into fragment ions generated in the MALDI ion source and the QIT cell. The ISD spectra obtained with TOF MS (Figures 1 and 3) contain fragment ions generated within the delayed extraction time (53 ns) corresponding to the residence time in the MALDI ion source. The observed fragment c-, z′-, z-ANL, y-, and w-ions (Figure 1 and Table 1) originate from direct ISD and follow reactions occurring within 53 ns in the MALDI ion source. The direct ISD reactions generate intense c-ions, whereas the subsequent reactions produce weak peaks of z′-, z-ANL-, w-, and y-ions (Scheme 2). Although the y-ions are generally observed together with b-ions in the low-energy CID spectra of peptides [15], the y-ions which are often observed together with z′-ions in the MALDI-ISD spectra of intact proteins [16] have been explained by radical-initiated fragmentation (RIF) [7]. It is also interesting that the ISD occurring within 53 ns in the MALDI ion source does not result in the loss of a phosphate group from either β-casein and its model peptide (Figures 1 and 3). This indicates that the ISD events that occur within 53 ns in the ion source are not a result originated from vibronic energy randomization resulting in an ergodic cleavage as pointed out by Lennon et al. [17], and that the ISD is mainly characterized by a prompt non-ergodic reaction without hydrogen scrambling [18, 19]. It is also of importance to recognize that the MALDI-ISD is essentially an RIF reaction of hydrogen-abundant neutral species [M + H]• and occurs independently of the ionization (protonation/deprotonation) process [20]. Considering that RIF such as the McLafferty rearrangement reaction [21–23] occurs within the 10–14 to 10–12 s timeframe, which corresponds to the frequency factor υ in Equation 1, it is expected that unpaired electrons in radical species extremely lower the threshold energy for cleavage reactions E 0 in Equation 1. The direct ISD in MALDI supports this hypothesis.
In contrast, the ISD spectra obtained from QIT-TOF MS (Figures 2 and 4) show characteristic peaks corresponding to fragment ions originating from the loss of neutral phosphoric acid from b-, c-, y-, and analyte ions [M + H]+ and [M – H]–. These fragment ions are produced within the residence timeframe of 140 ms through cooling processes in the QIT cell. Furthermore, many b- and y-ions were observed compared with the levels observed in ISD spectra with TOF-MS (Figures 1 and 3). The b- and y-ions in low-energy CID spectra are explained by the “mobile proton,” “proline (Pro) effect,” “asparagine (Asn)/glutamine (Gln) effect,” and “aspartic acid (Asp)/glutamic acid (Glu)/cysteine (Cys) effect” [8, 15]. The striking peaks of b7 and b8 ions observed in both Figures 2 and 4 can be explained by the Asn/Gln effect and the Pro effect, respectively. The peaks of y21 ion peak (Figure 2) and y10, y14, and y24 ion peaks (Figure 4) can be explained by the Pro effect. It is suggested, therefore, that the b-ions observed in Figures 2 and 4 may originate from unexpected cleavages attributable to the effects of internal energy activation and/or long residence time in the QIT cell, and that the y-ions originate from both ISD in the MALDI ion source and fragmentation in the QIT cell. The y-ions observed in Figures 1 and 3 originate from the prompt radical-initiated ISD and resultant reactions in the MALDI ion source. It is also of importance to recognize that the formation of b/y-ions and ions that have lost phosphoric acid (Figures 2 and 4) may originate from the injection of the source-generated ions into the bath gas environment of QIT cell.
Regarding the internal energy activation of the trapped ions in the QIT cell described above, there are limited reports. Black et al. reported that the collisional cooling of the trapped ions with helium gas resulted in the internal energy cooling, which inhibits bond dissociations [24]. The collisional cooling with helium gas lowers the translational energy T of the analyte and fragment ions, rapidly damping down (within several tens ms) their ion oscillations in the QIT cell [25]. Although there is no evidence for the energy conversion from T to vibrational energy V of the trapped ions through collisional cooling processes, it is reasonable to assume that ultra-low-energy multiple collisions with a long residence time in the QIT cell results in the energy conversion T to V, although just little momentum transfer is expected [26]. The unexpected cleavages to form b/y-ions observed here may originate from a combination of several effects of very little energy conversion T to V, long residence timeframe (140 ms) in the QIT cell, and specific amino acid effects described above. It is also a possible effect to explain that the energy conversion T to V may occur due to the injection of the source-generated ions into the QIT cell keeping the bath gas. Such effects would open lower energy windows for ergodic fragmentation reactions leading to the formation of b/y-ions and ions that have lost phosphoric acid.
It is well known that the loss of phosphate groups (H3PO4, 98 u) from the precursor ions occurs in CID experiments of phosphorylated peptides [5, 6]. It is reasonable to consider that positive and negative fragment b- and y-ions and ions that have lost a phosphate group observed in Figures 2 and 4 are produced by low-energy collisional cooling processes with a long residence timeframe in the QIT cell, whereas c- and z′-ions observed in the ISD spectra correspond to the ion species surviving degradation in the QIT cell. The fragment ions classified into fragmentation type, reaction space, and timeframe are summarized in Table 3.
In order to examine the product ion types originating from the degradation of c-, z′-, and y-ions stored in the QIT cell, low-energy CID experiments performed with argon gas for 30 ms were undertaken using the MALDI-QIT-TOF MS instrument. The product ions observed in positive- and negative-ion CID spectra of the c16 (Figure 4), z′ 10 (Figure 2), and y11 ions (Figure 2) are summarized in Table 4. The neutral species lost from b-, c-, z′-, and y-ions can be assigned as 18 (H2O), 17 (NH3), 98 (H3PO4), 30 (CH2O originating from Ser) and 44 (C2H4O originating from Thr). It is clear that all the precursor c-, z′- and y-ions can produce b- and y-ions in the low-energy CID experiments (data not shown). The successive neutral lost fragment ions are characteristic features in negative-ion CID [8].
Loss of Phosphoric Acids from Analyte and Fragment Ions
Both positive- and negative-ion ISD spectra of the phosphorylated model peptide obtained from QIT-TOF MS show fragment ions originating from the loss of phosphoric acid (H3PO4, 98 u) from analyte ions [M + H]+ at m/z 3605.6 and [M – H]– at m/z 3603.6, and from fragment y-ions (Figure 2). This indicates that the loss of phosphoric acid from the analyte and fragment ions occurs independently of both positive- and negative-charges (i.e., due to “charge-remote β-elimination (Scheme 3a)” [27]. In contrast, the ISD spectra obtained from TOF MS do not show any fragments originating from the loss of phosphoric acid (Figures 1 and 3). Furthermore, the formation of w-ions observed in both positive- and negative-ion ISD spectra obtained with TOF MS (Figure 1 and Table 1) can be explained by a prompt RIF reaction within 53 ns in the MALDI ion source (Scheme 3b) [14]. Comparison of the QIT-TOF MS and TOF MS data described above indicates that significant phosphoric acid loss from analyte ions [M + H]+ and [M – H]– in the QIT-TOF MS may be due to a long residence timeframe (140 ms) leading to a slow CRF ergodic reaction (Scheme 3a).
Although the neutral loss of phosphoric acid from phosphorylated peptides is the most characteristic feature in low-energy CID experiments [5, 6], interestingly, high-energy TOF/TOF CID [6] and electron capture dissociation (ECD)/electron transfer dissociation (ETD) experiments [28–30] give few product ions corresponding to the loss of phosphoric acid. In the case of TOF/TOF experiments, the timeframe (μs) of passing through the collision cell may be shorter than that for ergodic degradation through sufficient energy randomization. In contrast, the ECD/ETD experiments are performed using FTICR instruments that require a timeframe of several ms for the electron capture process. The lack of neutral loss of phosphoric acid from phosphorylated peptide ions in FTICR-ECD/ETD experiments may be due to the absence of collisional cooling gas. Thus, the internal energy E in Equation 1 deposited in FTICR cell may be smaller than the activation energy E 0 for the loss of neutral phosphoric acid.
Conclusions
The fragment ions observed in MALDI-ISD spectra of the phosphorylated protein β-casein and its model peptide were compared using two mass spectrometers with different ion optics, namely TOF MS and QIT-TOF MS, which differ in the timeframe for detecting analyte and fragment ions generated in the MALDI ion source. The QIT-TOF MS with a long timeframe (140 ms) gave c-, z′-, z-ANL, y-, and b-ions and ions that had lost phosphoric acid [M/c/z′/y/b – 98n, n = 1, 2], whereas the MALDI-TOF MS with a short timeframe (μs) only showed c-, z′-, z-ANL, y-, and w-ions. The formation of the ions generated in the MALDI source (c-, z′-, z-ANL, y-, and w-ions) can be explained by a prompt radical-initiated fragmentation of hydrogen-abundant radical ions [M + H + H]+•and [M + H – H]–• within 53 ns corresponding to the delayed extraction time. These source-generated ions can be detected by TOF MS without further degradation. In the case of QIT-TOF MS, on the other hand, the source-generated ions can be further degraded in the QIT cell before the ion detection. The QIT-TOF MS showed further characteristic degraded fragments such as ions that had lost phosphoric acid [c/z′/y/b – 98n, n = 1, 2] and b-ion, which can be explained by “charge-remote fragmentation (CRF)” [27] and low-energy CID mechanisms (“mobile proton,” “Pro effect,” and “Asn/Gln effect” [8, 15]), respectively. The identity of the further degraded fragment ions was confirmed by positive- and negative-ion low-energy CID experiments on the source-generated ions such as c-, z′-, and y-ions. The positive-ion CID spectra showed b/y-ions and ions that had lost phosphoric acid [b/y – 98], whereas negative-ion CID resulted in the loss of neutrals H2O, NH3, CH2O (from Ser), C2H4O (from Thr), and H3PO4 from the precursor and product b/y/c/z′-ions. Although the formation of b/y-ions observed in QIT-TOF MS can be characterized by low-energy CID mechanisms, it has been suggested that y-ions can be formed from direct and/or following ISD reactions, which are a prompt radical-initiated fragmentation within 53 ns in the MALDI ion source. The loss of phosphoric acid (98 u) from analyte and fragment ions takes place independently of positive and negative charges. The cooling process during the long residence timeframe (140 ms) of the trapped ions in the QIT cell or the injection of the source-generated ions into the QIT cell keeping the bath gas might result in an increase of very little internal energy E through energy conversion and slow ergodic fragmentations such as neutral losses. Further study is needed for clarifying the energy conversion T to V through the collisional cooling in the QIT cell or the injection of the ions into the QIT cell.
References
Karas, M., Bachmann, D., Bahr, U., Hillenkamp, F.: Matrix-assisted ultraviolet laser desorption of non-volatile compounds. Int. J. Mass Spectrom. Ion Process. 78, 53–68 (1987)
Tanaka, K., Waki, H., Ido, Y., Akita, S., Yoshida, Y., Yoshida, T.: Protein and polymer analyses up to m/z 100,000 by laser ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2, 151–153 (1988)
Whitehouse, C.M., Dreyer, R.N., Yamashita, M., Fenn, J.B.: Electrospray interface for liquid chromatographs and mass spectrometers. Anal. Chem. 57, 675–679 (1985)
Fenn, J.B., Mann, M., Meng, C.K., Wong, S.F., Whitehouse, C.M.: Electrospray ionization for mass spectrometry of large biomolecules. Science 246, 64–71 (1989)
Asakawa, D., Takayama, M.: Mass spectrometric characterization of phosphorylated peptides using MALDI in-source decay via redox reactions. J. Mass Spectrom. 47, 180–187 (2012)
Markoutsa, S., Surun, D., Karas, M., Hofman, B., Steinhilber, D., Sorg, L.: FEBS J. 281, 1931–1947 (2014)
Asakawa, D., Calligaris, D., Zimmerman, T.A., De Pauw, E.: In-source decay during matrix-assisted laser desorption/ionization combined with the collisional process in an FTICR mass spectrometer. Anal. Chem. 85, 7809–7816 (2013)
Takayama, M., Sekiya, S., Iimuro, R., Iwamoto, S., Tanaka, K.: Selective and nonselective cleavages in positive and negative CID of the fragments generated from in-source decay of intact proteins in MALDI-MS. J. Am. Soc. Mass Spectrom. 25, 120–131 (2014)
Johnson, R.S., Martin, S.A., Biemann, K.: Collision-induced fragmentation of [M + H]+ ions of peptides. Side chain specific sequence ions. Int. J. Mass Spectrom. Ion Process. 86, 137–154 (1988)
Steinfeld, J.I., Francisco, J.S., Hase, W.L.: Chemical kinetics and dynamics, 2nd edn, pp. 340–343. Prentice-Hall, Inc, New Jersey (1998)
Zirrolli, J.A., Murphy, R.C.: Low-energy tandem mass spectrometry of the molecular ion derived from fatty acid methyl esters: a novel method for analysis of branched-chain fatty acids. J. Am. Soc. Mass Spectrom. 4, 223–229 (1993)
Vidavsky, I., Chorush, R.A., Longevialle, P., McLafferty, F.W.: Functional group migration in ionized long-chain compounds. J. Am. Chem. Soc. 116, 5865–5872 (1994)
Takayama, M.: A terminal product ion in the fragmentation of methyl stearate under electron ionization conditions. J. Mass Spectrom. Soc. Jpn. 46, 139–142 (1988)
Takayama, M., Nagoshi, K., Iimuro, R., Inatomi, K.: Access of hydrogen-radicals to the peptide-backbone as a measure for estimating the flexibility of proteins using matrix-assisted laser desorption/ionization mass spectrometry. Int. J. Mol. Sci. 15, 8428–8442 (2014)
Paizs, B., Suhai, S.: Fragmentation pathways of protonated peptides. Mass Spectrom. Rev. 24, 508–548 (2005)
Takayama, M.: Susceptible region of myoglobins to in-source decay using matrix-assisted laser desorption/ionization coupled with delayed extraction reflectron time-of-flight mass spectrometer. J. Mass Spectrom. Soc. Jpn. 50, 304–310 (2002)
Lennon, J.J., Walsh, K.A.: Locating and identifying posttranslational modifications by in-source decay during MALDI-TOF mass spectrometry. Protein Sci. 8, 2487–2493 (1999)
Bache, N., Rand, K.D., Roepstorff, P., Jϕrgensen, T.J.D.: Gas-phase fragmentation of peptides by MALDI in-source decay with limited amide hydrogen (1H/2H) scrambling. Anal. Chem. 80, 6431–6435 (2008)
Rand, K.K., Bache, N., Nedertoft, M.M., Jϕrgensen, T.J.D.: Spatially resolved protein hydrogen exchange measured by matrix-assisted laser desorption ionization in-source decay. Anal. Chem. 83, 8859–8862 (2011)
Takayama, M., Tsugita, A.: Does in-source decay occur independent of the ionization process in matrix-assisted laser desorption? Int. J. Mass Spectrom. Ion Process. 181, L1–L6 (1998)
McLafferty, F.R.: Mass spectrometric analysis molecular rearrangement. Anal. Chem. 31, 82–87 (1959)
Takayama, M.: Metastable McLafferty rearrangement reaction in the electron impact ionization of stearic acid methyl ester. Int. J. Mass Spectrom. Ion Process. 144, 199–204 (1995)
Becky, H.D., Hey, H., Levsen, K., Tenschert, G.: Study of the kinetics of fast unimolecular decomposition processes and of organic rearrangement reactions by field ionization mass spectrometry. Int. J. Mass Spectrom. Ion Phys. 2, 101–123 (1969)
Black, D.M., Payne, A.H., Glish, G.L.: Determination of cooling rates in a quadruple ion trap. J. Am. Soc. Mass Spectrom. 17, 932–938 (2006)
He, M., Guo, D., Feng, Y., Xiong, X., Zhang, H., Fang, X., Xu, W.: Realistic modeling of ion-neutral collisions in quadrupole ion traps. J. Mass Spectrom. 50, 95–102 (2015)
McLuckey, S.A.: Principle of collisional activation in analytical mass spectrometry. J. Am. Soc. Mass Spectrom. 3, 599–614 (1992)
Boersema, P.J., Mohammed, S., Heck, A.J.R.: Phosphopeptide fragmentation and analysis by mass spectrometry. J. Mass Spectrom. 44, 861–878 (2009)
Stensballe, A., Jensen, O.N., Olsen, J.V., Haselmann, K.F., Zubarev, R.A.: Electron capture dissociation of singly and multiply phosphorylated peptides. Rapid Commun. Mass Spectrom. 14, 1793–1800 (2000)
Shi, S.D.-H., Hemling, M.E., Carr, S.A., Horn, D.M., Lindh, I., McLafferty, F.W.: Phosphopeptide/phosphoprotein mapping by electron capture dissociation mass spectrometry. Anal. Chem. 73, 19–22 (2001)
Molina, H., Horn, D.M., Tang, N., Mathivanan, S., Pandey, A.: Global proteomic profiling of phosphopeptides using electron transfer dissociation tandem mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 104, 2199–2204 (2007)
Acknowledgment
M.T. gratefully acknowledges the support from the Creation of Innovation Centers for Advanced Interdisciplinary Research Area Program in the Special Coordination Fund for Promoting Science and Technology.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sekiya, S., Nagoshi, K., Iwamoto, S. et al. Timeframe Dependent Fragment Ions Observed in In-Source Decay Experiments with β-Casein Using MALDI MS. J. Am. Soc. Mass Spectrom. 26, 1588–1598 (2015). https://doi.org/10.1007/s13361-015-1173-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-015-1173-3