Abstract
RNA polymerase sigma factors are indispensable in the process of bacterial transcription. They are responsible for a given gene’s promoter region recognition on template DNA and hence determine specificity of RNA polymerase and play a significant role in gene expression regulation. Here, we present a simple and unified protocol for purification of all seven Escherichia coli RNA polymerase sigma factors. In our approach, we took advantage of the His8-SUMO tag, known to increase protein solubilization. Sigma factors were first purified in N-terminal fusions with this tag, which was followed by tag removal with Ulp1 protease. This allowed to obtain proteins in their native form. In addition, the procedure is simple and requires only one resin type. With the general protocol we employed, we were able to successfully purify σD, σE, σS, and σN. Final step modification was required for σF, while for σH and σFecI, denaturing conditions had to be applied. All seven sigma factors were fully functional in forming an active holoenzyme with core RNA polymerase which we demonstrated with EMSA studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In their natural environment, bacterial cells are exposed to constantly changing conditions to which they should react quickly in order to survive. This adaptation usually takes place at the step of gene expression regulation, allowing cells to change their metabolism according to conditions and resources available. Such regulation may involve transcriptional factors, second messengers (e.g., cAMP, (p)ppGpp), or simply RNA polymerase (RNAP) sigma factors.
Sigma factors are responsible for specificity of the RNAP holoenzyme, as they direct it towards gene promoter regions and allow the whole complex to bind the DNA and subsequently to initiate gene transcription. The core RNAP enzyme (i.e., without a sigma factor) is composed of two α subunits, and one of each, β, β’, and ω, and it does not bind DNA in a selective manner [for review see: Murakami and Darst 2003; Murakami 2015].
In Escherichia coli, there are seven sigma factors, where the major or “housekeeping” factor is σD (also called σ70; the numbers in sigma factor names come from approximate molecular weight of a given factor, in kDa). This factor is responsible for directing gene transcription under normal growth conditions. The other six sigma factors are often called “alternative” as they participate in gene expression regulation only under specific conditions [for review see: Österberg and del Peso-Santos 2011; Feklístov et al. 2014; Paget 2015; Davis et al. 2017; Helmann 2019]. For example, two sigma factors were found to be active under heat shock: σH (σ32) is already active under moderate heat shock conditions [Arsène et al. 2000], while σE (σ24) is active under extreme heat shock when denatured proteins accumulate in the periplasm or under conditions that cause periplasmic stress [Raina et al. 1995; Rouvière et al. 1995]. On the other hand, σF (σ28) controls expression of genes encoding flagella and those necessary for chemotaxis [Anderson et al. 2010], while σN (σ54) is active for example under nitrogen stress conditions [Shingler 2011]; yet σS (σ38) directs gene expression when cells are in the stationary phase of growth and is responsible for the general stress response [Battesti et al. 2011; Gottesman 2019]. The last sigma factor, σFecI (σ19) controls expression of Fe3+ transport genes [Van Hove et al. 1990; Angerer et al. 1995] and so far is the most obscure, as only one promoter has been identified to date to depend on this sigma factor [Angerer et al. 1995; Maeda et al. 2000; Shimada et al. 2017].
In order to study specific mechanisms governing transcription, in vitro methods are often employed. There, the reaction components must be very well defined, and the protein purity and proper folding is of the utmost importance. To date, many different approaches have been applied to purify E. coli sigma factors, very often involving purification from inclusion bodies (e.g., Angerer et al. 1995; Anthony et al. 2003; Enz et al. 2000; Maeda et al. 2000; Shikalov et al. 2019] and/or different resin types, such as those for ion exchange chromatography or size exclusion chromatography (e.g., Anthony et al. 2003; Liberek et al. 1992; Shikalov et al. 2019]. These protocols often require expensive resins or equipment that is not always readily available (e.g., MonoQ and the HPLC system). In addition, protein refolding from inclusion bodies may be problematic, as the proteins first aggregate in these dense structures and then are denatured under harsh conditions, followed by renaturation that may or may not be fully successful. On the other hand, there are protocols that involve the use of His-tag and immobilized metal affinity chromatography (IMAC), in combination with or without additional columns, where upon the final purification step the His-tag may or may not be removed by employing specific proteases, such as thrombin protease (in case of proteins expressed with the thrombin cleavage site) [Anthony et al. 2003; Lu et al. 2023; Obrist et al. 2009; Becker et al. 1999]. However, these protocols are not applicable to all E. coli sigma factors, while in case of studies comparing their activity, it would be ideal to purify all of them under the same conditions.
Here, we report on a simple protocol that can be employed for purification of all seven E. coli sigma factors. We took advantage of the SUMO tag, known to increase protein solubilization and decrease their aggregation [Marblestone et al. 2006]. In addition, such a tag was shown to enhance protein overexpression and decrease degradation when fused to the N-terminus of a given protein [Panavas et al. 2009; Peroutka Iii et al. 2011].
Indeed, the employed procedure allowed us to obtain all overproduced sigma factors in the soluble form, without the need for extraction from inclusion bodies. In addition, the SUMO tag was fused with His8 tag thanks to which all proteins could be purified by IMAC with the use of only one type of resin. Gravity flow chromatography that does not require any special equipment is employed. Upon His8-SUMO tag removal with specific SUMO protease, all sigma factors obtained are in their native form, i.e., without any additional tags. Finally, we demonstrate with the use of EMSA technique that the obtained factors are able to form functional RNAP holoenzymes.
Materials and methods
Bacterial strains and plasmids
Bacterial strains used in this study were E. coli MG1655 (ATTC #700926; used for sigma factor genes’ or their respective dependent promoter regions’ PCR amplification), E. coli DH5α (used for transformation with constructed plasmids and for plasmid maintenance), or E. coli BL21 (λDE3) (Novagen®; used for protein overexpression and purification). KP517 strain [Dylewski et al. 2019] was used for σ70 and σ24 - dependent promoter region amplification (this strain carries a modified greA promoter region that previously allowed us to study transcription by RNAP holoenzyme with the two mentioned sigma factors on the same DNA fragment).
All plasmids were constructed based on a pET28a derivative (pKB1, this work) in which His8-SUMO-tag has been inserted, followed by a convenient NdeI site. The genes of interest are cloned between NdeI and Bpu1102I sites, so the cloning/expression region’s structure is as follows: T7 promoter – lac operator – XbaI site – His8 – SUMO tag – NdeI site – gene of interest – Bpu1102I site – T7 terminator (original pET28a sequences are underlined) (Fig.1A). Sequence of the His8-SUMO tag has been derived from pCIOX (a gift from Dr. Andrea Mattevi; Addgene plasmid # 51300).
Overproduction of E. coli sigma factors. A Schematic representation of key elements in the plasmid used for sigma factor overproduction. As an example, plasmid used for σS overexpression is shown. SnapGene software was used. B Example of IPTG induction of sigma factor overproduction. σS overexpression is visualized by Coomassie blue-stained SDS-PAGE (10–20%). M, Perfect Tricolor protein ladder (Eurx); 1, uninduced cell lysate, 2–5, cell lysate obtained after 1, 2, 3, and 4-h induction with 1 mM IPTG. Equal amounts of lysate were loaded in each lane. Molecular weights of the marker bands are indicated on the left of the gel
Plasmids used in this study are listed in Table S1, and primers used for PCR amplification of the sigma subunit genes are listed in Table S2. All DNA constructs obtained were verified by sequencing (Macrogen Europe). Constructed plasmids are available upon request.
Protein overexpression and purification
In general, the protocol for sigma factor purification was as follows. The cells were grown at 32 °C in 250 ml of LB (supplemented with kanamycin, 30 μg/ml) until OD600~0.4, upon which protein overexpression was induced by addition of IPTG to 1 mM. The cultivation was continued for 3.5 h, after which the cells were harvested by centrifugation (3200 x g, 30 min, 4 °C) and stored at -20 °C until further use. The cell pellets were resuspended in 15 ml of ice-cold lysis buffer (20 mM Tris-Cl pH 8.0, 500 mM NaCl, 5% glycerol, 0.1 mM EDTA, 5 mM imidazole, 0.5 mM β-mercaptoethanol; LysB) supplemented with EDTA-free protease inhibitor cocktail (Thermo Fisher Scientific) and lysozyme (0.1 mg/ml) and incubated on ice for 30 min with gentle mixing every 10 min or so. Following sonication (on ice, 5 s on and off cycles, 2.5 min total sonication time; Vibra-Cell apparatus (Sonics)) and centrifugation (20,000 x g, 30 min, 4° C), the supernatants were applied on a BioRad disposable column pre-loaded with 2.5 ml of HisPur Ni-NTA resin (Thermo Fisher Scientific), pre-equilibrated with 15 ml of LysB. Resin was then washed twice with 30 ml of the wash buffer (same as LysB but containing 20 mM imidazole; WB-1), followed by His8-SUMO-tagged protein elution with three applications of 3 ml of the elution buffer (same as lysis buffer but with 250 mM imidazole; EB-1).
Fractions containing desired His-tagged protein (6–9 ml) were then pooled, placed in Slide-A-Lyzer cassettes (10 kDa cutoff; Thermo Fisher Scientific), and dialyzed overnight at 4°C in 900 ml of the following buffer: 20 mM Tris-HCl pH, 250 mM NaCl, and 5 % glycerol (DB-1); this was followed by dialysis in a fresh change of the same buffer for 1 h. The samples were removed and incubated with the in-house purified S. cerevisiae His-tagged Ulp1 SUMO protease (final concentration 10 μg/ml) [Sobala et al. 2019], incubated for 45 min at 4 °C on a rocker, and then applied on a BioRad disposable column pre-loaded with 1.5 ml of HisPur NiNTA resin, pre-equilibrated with 9 ml of DB-1. Flow-through fractions were collected, and the column was washed with 4 ml of the same buffer to elute the unbound proteins with removed His8-SUMO tag. These were then pooled and concentrated with the use of Amicon-15 Ultrafiltration devices (10 kDa molecular weight cut-off (MWCO)), and then the buffer was exchanged for 1 x TGED (20 mM Tris-Cl pH 8.0, 20% glycerol, 500 mM NaCl, 1 mM EDTA, 1 mM DTT) with the same devices. Finally, glycerol was added to 50%, and protein concentration was assessed with Qubit Protein Assay (Thermo Fisher Scientific).
All procedures were carried out at 4 °C with ice-cold buffers. At each step, the culture, cells and protein fractions were monitored for appropriate protein content by SDS-PAGE (10-20% Novex Tris-Glycine gels, Thermo Fisher Scientific) and Coomassie blue staining [Lawrence and Besir 2009].
For σF, σFecI, and σH purification, modified protocols were applied, as discussed in the Results and Discussion section. The detailed protocols are provided below.
For σF purification, the final protein prep in 1 x TGED was reapplied on a column pre-loaded with 1.5 ml of the HisPur NiNTA resin (pre-equilibrated with 9 ml of the 1x TGED buffer), and only the flow-through fraction was collected and used in further procedures.
For σFecI, the first steps were the same as for other sigma factor purification, except that WB-1 contained 1 M NaCl. Next, denaturing buffers were applied. First, the resin was washed with 10 ml of 6 M guanidine hydrochloride, 20 mM imidazole, 20 mM Tris-HCl pH 8.0, and 500 mM NaCl (WB-2). Then, His-tagged proteins were eluted with a buffer the same as WB-2 but containing 250 mM imidazole (10 ml; EB-2), placed in Slyde-A-Lyzer cassettes, and dialyzed for 2 h against 1 L of the following buffer: 3 M urea, 20 mM Tris-Cl pH 8.0, 250 mM NaCl, and 5 % glycerol (DB-2). This was followed by 2 h dialysis against buffers containing decreasing urea concentrations (the same as DB-2 but containing 2 M and 1M urea (DB-3 and DB-4, respectively)), and finally overnight dialysis against DB-1. Next steps (His8-SUMO-tag cleavage, passing through a column, buffer exchange, and sample concentration) were the same as for the other sigma factors.
For σH purification, a modified wash procedure was followed by wash and dialysis under denaturing conditions. Upon applying cell lysate to the column, the resin was washed with 30 ml of WB-1supplemented with 5 mM ATP and 5 mM MgCl2 (WB-3), followed by 30 ml of WB-3 supplemented with 0.1 μg/ ml denatured proteins. Denatured proteins were obtained from 10 ml of E. coli MG1655 overnight culture (OD600~5.0, grown in LB); the cells were centrifuged (5,000 x g, 5 min), suspended in 2 ml of WB-1, sonicated (10 s on, 10 s off, 5 min total sonication time), centrifuged (14,000 x g, 10 min), incubated at 65 °C for 10 min, and re-centrifuged; protein concentration was estimated by A280 measurements (Nanodrop, Thermo). The resin was then washed with 30 ml of WB-3. The next steps were the same as for σFecI purification under denaturing conditions (10 ml wash with WB-2, and then elution (EB-2), dialysis against buffers containing decreasing urea concentrations (DB-2, DB-3, and DB-4), and finally against DB-1). Next steps (His8-SUMO-tag cleavage, passing through a column, buffer exchange and sample concentration) were the same as for the other sigma factors.
Electrophoretic mobility shift assays (EMSA)
These assays were performed with PCR amplified, Cy5-end-labeled dsDNA linear templates containing promoter regions recognized by a given sigma factor. Primers used are listed in Table S3. And 20 μl reactions containing 30 nM E. coli RNA polymerase core enzyme (Epicentre) and 300 nM or 600 nM purified sigma factor (10 or 20 molar excess over the core, respectively) were assembled in a binding buffer (50 mM Tris-HCl pH 8.0, 50 mM KCl, 10 mM MgCl2, 10 mM β-mercaptoethanol, 2.5% glycerol) and incubated for 20 min at 37 °C to allow reconstitution of the holoenzyme. Then, Cy5-labeled DNA templates (10 nM, final) were added, and incubation was continued for another 15 min to allow DNA binding. Next, samples were loaded on 4% native polyacrylamide running gels (acrylamide/bisacrylamide ratio of 19:1) and electrophoresed in 0.5 x TBE buffer for 70 or 85 min (depending on the DNA length) at 100 V, room temperature. Detection of the Cy5–labeled DNA was performed using Typhoon scanner (GE Healthcare).
Results and discussion
Cloning of plasmids for overexpression of sigma factors
Appropriate sigma factor genes were cloned into a pET28a derivative containing His8-SUMO tag between NdeI and Bpu1102I restriction sites (see Materials and Methods for details and Fig. 1A). Gene expression was driven from a T7 RNA polymerase dependent promoter. DNA fragments for cloning were amplified from wild type E. coli chromosomal DNA with several exceptions (described below).
Since rpoN (σN encoding gene) contains a Bpu1102I site close to its 5’ terminus, an amplification primer was designed to introduce a silent mutation destroying that site. Similarly, rpoH (σH encoding gene) contains a Bpu1102I site at its 3’ terminus. In this case, a silent mutation was introduced by amplification of two separate fragments by PCR and then combining them into one fragment in a final PCR reaction. In case of rpoD (σD encoding gene), which contains both NdeI and Bpu1102I sites in its native sequence, a DNA fragment carrying silent mutations at these sites was obtained commercially (GeneArt service, Thermo Fisher Scientific; sequence provided in Supplementary Data) and used as template for subsequent PCR amplification and cloning. All DNA constructs obtained were verified by sequencing.
Sigma factor purification: general protocol
The plasmids obtained were introduced into BL21(λDE3) strain carrying T7 RNAP gene under an IPTG inducible promoter. To verify expression, appropriate strains were first grown in 20 ml of LB at 32 °C to OD600~0.4 upon which IPTG was added to 1 mM and growth was continued for 4 h. Cell samples were removed at every hour, and protein content was monitored by SDS-PAGE. In most cases, overexpressed protein accumulation reached saturation at 3–4 h after induction (Fig. 1B and data not shown). Thus, for large-scale sigma factor overproduction, 3.5-h induction was used in general.
In our first attempts to purify E. coli RNAP sigma factors, we employed a standard procedure for His8-SUMO tagged proteins used in our laboratory, based on ion metal affinity chromatography (IMAC) (e.g., [Sobala et al. 2019]); see Materials and Methods for details). Briefly, the cells are grown in 250 ml of LB to OD600~0.4 at 32 °C, induced with IPTG, and then collected by centrifugation and frozen. The cell pellets are then resuspended in a lysis buffer, sonicated, and supernatants are applied to a column preloaded with Ni2+-NTA resin. Upon several washes with a buffer containing low concentration of imidazole (20 mM), the desired proteins are eluted with a buffer containing high imidazole concentration (250 mM), and dialyzed overnight in a buffer that allows for Ulp1 SUMO protease activity, which is used at a subsequent step to remove the His8-SUMO tag from purified sigma factors. Since the protease itself is also His-tagged, passing the whole sample through another Ni2+-NTA resin column allows for removal of both, the cleaved His8-SUMO tag, and the protease. In addition, if the prep is contaminated with proteins that interact with the resin but that were not removed at previous steps (i.e., they were not removed by washes of the first column), that second column allows for such clean-up. The flow-through sample is then collected, the column is washed with a small volume of the same buffer to elute unbound proteins present in the column dead volume, the pooled sample is concentrated, and the buffer is exchanged for sigma factor storage buffer (1 x TGED).
This procedure worked very well for overexpression and purification of σD, σE, σS, and σN (Fig. 2). However, in case of σF, σH, and σFecI, some modifications had to be introduced since the preps obtained by the general procedure described above contained substantial amounts of co-purified other proteins (Fig.3 and Fig.S1–2).
Purification of sigma factors by employing the general purification protocol. A σD, B σE, C σN, and D σS, as visualized by Coomassie blue-stained SDS-PAGE (10–20%). M, Perfect Tricolor protein ladder (Eurx); 1, uninduced cells; 2, lysate obtained after 3.5-h IPTG induction; 3, sample obtained after sonication; 4, supernatant after centrifugation step; 5, flow-through upon column loading with supernatant; 6, flow-through after applying first portion of wash-buffer; 7, flow-through after applying second portion of wash-buffer; 8, elution, first fraction; 9, elution, second fraction; 10, elution, third fraction; 11, sample after dialysis; 12, after Ulp1 addition; 13, flow-through after applying sample to the column; 14, 2 μl of the sample concentrated with the Amicon filtration device (10 MWCO); 15, 5 μl of the same sample as in 14. Molecular weights of the marker bands are indicated on the left of each gel
Purification of σF by employing modified general purification protocol. Lanes marked as M and 1–15 are the same as described in Fig.2. Lane 13’, flow-through upon dilution of sample from lane 15 and another loading on the column; 14’, 2 μl of the sample run in 13’ but concentrated with the Amicon filtration device (10 MWCO); 15’, 5 μl of the same sample as in 14’. See Materials and Methods for details. Molecular weights of the marker bands are indicated on the left of each gel
Purification of σF
Here, many protein contaminants were still present after the second column and His8-SUMO tag removal (Fig. 3, lanes 13–15). We suspected that some of these contaminants were enriched after column dead-volume wash and thus we applied a rather simple remedy—the final sample was diluted in a large volume (12 ml) of the 1 x TGED buffer, and the whole sample was re-applied onto fresh Ni2+-NTA resin. Only the flow-through fraction was this time collected and then concentrated. As shown in Fig. 3 (lanes 13’–15’), most of the high-molecular weight proteins were removed. Although this resulted in σF losses, the sample purity was enhanced.
σH and σFecI purification
These two sigma factors were the most difficult to purify due to the prep’s high contamination with co-purified proteins when applying the general purification procedure (Fig. S1A and S2).
In case of σH, we suspected that the contaminants were in large part chaperone proteins (such as DnaK, DnaJ, GrpE, and/or GroEL/GroES) that bind σH under non-heat shock conditions to prevent its activity [Arsène et al. 2000; Guisbert et al. 2004]. Such interactions were reported in other papers dealing with σH purification [e.g., Liberek et al. 1992]. On the other hand, it was reported that chaperone (DnaK) contamination of a given protein prep could be overcome by adding ATP, Mg2+, and denatured proteins to the wash buffer [Rial and Ceccarelli 2002]. These allow for the chaperone unbinding from σH and binding to their preferred substrates, i.e., the added denatured proteins. In our case, this procedure helped in removing some, but not all of the co-purified proteins (Fig. S1B).
We thus decided to undertake a different approach, where in addition to the steps just described, we applied denaturing conditions to unbind proteins that specifically and tightly interact with σH. Thus, 6M guanidine hydrochloride buffer was used to wash the resin with bound His-tagged proteins. The His8-SUMO tagged σH was then eluted under the same conditions and was brought back to its native state by subsequent dialysis in buffers with decreasing concentrations of another denaturant, i.e. urea. The ensuing steps (His8-SUMO tag cleavage and following procedures) were the same as in the general protocol. This way, we finally succeeded in obtaining pure σH protein preps (Fig. 4A).
Purification of σH and σFecI by employing denaturing conditions. A σH, B σFecI, as visualized by Coomassie blue-stained SDS-PAGE (10–20%). M and M’, Perfect Tricolor protein ladder (Eurx); 1, uninduced cells; 2, lysate obtained after 3.5-h IPTG induction; 3, sample obtained after sonication and subsequent centrifugation; 4, flow-through; 5, flow-through after applying first portion of wash buffer; 6, flow-through after applying second portion of wash buffer; 6’, flow-through after applying ATP/Mg2+/denatured proteins (for σH only, see Materials and Methods for details); 7, sample after dialysis; 8, after Ulp1 addition; 9, flow-through after applying sample to the column; 10, 5 μl of the sample concentrated with the Amicon filtration device (10 MWCO),; 11, 10 μl of the same sample as in 10. Molecular weights of the marker bands are indicated on the left of each gel
In case of σFecI, upon initial purification, we observed contamination with many proteins, among which four were predominant (~20 kDa, ~35 kDa, ~75 kDa, and ~180 kDa, Fig. S2). It is known that σFecI tightly interacts with FecR (35 kDa) which in turn also interacts with FecA (85 kDa) [Enz et al. 2000]. The apparent molecular weights of two of the co-purified proteins roughly correspond to FecR and FecA, although their identity remains unresolved.
In our first attempt at obtaining pure σFecI, we decided to increase salt concentration of the wash buffer to 1 M as this sometimes helps to remove unwanted interactions (see, e.g., [Sobala et al. 2019]). However, this did not yield any improvement (data not shown). Thus, we undertook the same approach as for σH, i.e., 6M guanidine hydrochloride was used to denature and unbind any potential proteins directly and tightly interacting with σFecI. In this case, the procedure turned out to be more successful, although some proteins still co-purified with this sigma factor (Fig. 4B).
Protein yields and sample purity
Protein yields for each sigma factor, calculated per 1 g of cell wet weight, are given in Table 1. To assess sample purity, preps of the final purified proteins were resolved by SDS-PAGE, which was followed by Coomassie blue-staining and densitometry (Fig. 5 and Table 1). It should be noted that it is known that sigma factors migrate differently in SDS-PAGE than it would be expected from their molecular weight [Helmann 2019]. For example, σD whose molecular weight is 70 kDa migrates around 90 kDa. Similarly, σN migrates much slower than expected for a 54 kDa protein.
Purified sigma factors visualized by Coomassie blue-stained SDS-PAGE (10–20%). M, Perfect Tricolor protein ladder (Eurx); D, σD (M.w. ~70 kDa); N, σN (M.w. ~54 kDa); S, σS (M.w. ~38 kDa); H, σH (M.w. ~32 kDa); F, σF (M.w. ~28 kDa); E, σE (M.w. ~24 kDa); FecI, σFecI (M.w. ~19 kDa). About 1.5 μg of each protein were loaded per lane. Molecular weights of the marker bands are indicated on the left of the gel
When taking into account the amount of protein obtained per 250 ml of IPTG induced cell culture, the highest amount was obtained for σD. E. coli culture overexpressing that sigma factor also grew the best after IPTG addition. On the other hand, when looking at the overall yield that takes into account cell mass (expressed as mg protein/1 g cell wet weight), it is evident that the highest yield was obtained for σE. Induction of that factor’s overproduction had a significantly detrimental effect on cell growth; however, σE production had proceeded nonetheless (data not shown). For five sigma factors (σD, σE, σH, σN, σS), sample purity was ≥ 93%, with the highest purity obtained for σD and σH (98%).
The least efficient was σF purification even though its overproduction did not severely impair growth. In order to obtain higher amounts of σF, larger cell culture volumes and/or longer induction times, growing cells at different temperatures, using different IPTG concentrations or inducing at different OD values should be used in the future and efforts taken to increase sample purity, such as employing a gel filtration or ion exchange column, instead of simply reloading sample on the nickel column after SUMO-tag removal. The same is true for σFecI whose purity was the lowest. Nevertheless, we deemed the obtained protein preps to be much increased in purity in comparison to the general protocol first used and decided to proceed with assessment of the activity of all sigma factor samples.
Assessment of the purified sigma factor activity
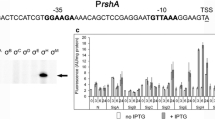
In order to assess whether the purified sigma factors obtained are active, i.e., whether they could form a functional holoenzyme with core RNAP, we employed EMSA assays. Here, each sigma factor was pre-incubated with core RNAP to form the holoenzyme, and then appropriate Cy5-labeled DNA template was added. Promoter regions specifically recognized by each sigma factor were chosen based on literature reports and were as follows: greAp1-p4 promoter region for σD and σE [Potrykus et al. 2010; Dylewski et al. 2019]; pflgM for σF [Park et al. 2001]; pgroE for σH [Nonaka et al. 2006; Wade et al. 2006]; p4relA for σN [Brown et al. 2014]; pxapA for σS [Maciag et al. 2011]; and pfecA for σFecI [Enz et al. 2003].
The samples were then loaded onto a running native PAGE, and a shift in the DNA’s electrophoretic migration indicated that a given DNA fragment was bound by proteins in the sample. As control, samples with core RNAP only or sigma factor only were also included. A shift in band migration occurring with the holoenzyme, but not with these factors alone, indicates that the interaction is specific to the whole complex, and thus the sigma factor under investigation is active in recognizing its corresponding promoter region when bound in the holoenzyme.
As can be seen in Fig. 6, all sigma factors purified here were able to form a functional holoenzyme with core RNAP. Band shifts were observed for all σ-core RNAP complexes, even for σF, whose yield was the least efficient and purity was only 82%. In addition, to our knowledge, the pflgM promoter region used here for σF holoenzyme was never mapped experimentally nor in vitro transcription data was reported to date for this promoter; its -10 and -35 regions were only inferred from DNA sequence [Park et al. 2001]. Here, we cannot confirm that these regions were attributed correctly; nevertheless, we do confirm that this region contains a σF-dependent promoter that is recognized by RNAP holoenzyme in vitro and that the σF obtained is active in promoter recognition when combined with RNAP core enzyme.
EMSA studies demonstrate that all purified sigma factors are capable of forming functional holoenzyme with core RNAP. Cy5-labeled promoter template was incubated with the E. coli core RNAP and/or with 10 or 20 fold molar excess of appropriate sigma factor over core RNAP, as indicated in the figure. Blue arrow and red asterisks denote positions of unbound DNA and major DNA-RNAP holoenzyme complexes, respectively
Another drawback could have been foreseen for σFecI whose yield was second to last and the prep was substantially contaminated with co-purified proteins (only 68% purity). As stated above, we speculate that one of those co-purified proteins might be FecR (M.w. 35 kDa). Interestingly, the σFecI interaction with FecR N-terminal domain was reported to be essential for σFecI functionality in transcription initiation [Ochs et al. 1995; Mahren et al. 2002]. It could be that fortuitously, the prep obtained might thus indeed contain the necessary σFecI partner, although not at stoichiometric concentrations, as judged by the Coomassie blue-stained SDS-PAGE gel (Fig. 4B and Fig. 5). However, that requires verification in future studies.
Concluding remarks
Here, we provide a simple and unified protocol for overproduction and purification of all seven E.coli RNAP sigma factors. The use of His8-SUMO tag had allowed obtaining all of these factors in the soluble form, unlike other protocols where some of these factors had to be extracted from inclusion bodies. We believe that the method presented is also elegant since it employs only one type of resin and after His8-SUMO tag removal by treatment with a specific protease (Ulp1) all sigma factors are in their native form. The established purification scheme is summarized in Fig. 7.
The general protocol employed proved successful for purification of four factors (σD, σE, σS, and σN) and upon slight modification for σF as well. In order to purify σH and σFecI away from proteins that tightly bind to them, denaturing conditions had to be applied. Still, all seven sigma factors obtained are active—they are able to form a functional holoenzyme with core RNAP, as we demonstrated by EMSA studies.
With the boom of high throughput techniques, it might seem that in vitro studies with purified factors to study transcription are obsolete. However, this is not the case, as dissecting specific molecular mechanisms at transcriptional level still requires obtaining pure proteins, whether it be for in vitro transcription, single molecule in vitro studies, mapping RNAP binding sites on DNA or establishing crystal structures of RNAP complexes. We thus believe that the protocols for purification of the seven E. coli sigma factors that we report here may be helpful to many others and in addition may be adapted for purification of sigma factors from other bacterial species as well.
References
Anderson JK, Smith TG, Hoover TR (2010) Sense and sensibility: flagellum-mediated gene regulation. Trends Microbiol 18(1):30–37. https://doi.org/10.1016/j.tim.2009.11.001
Angerer A, Enz S, Ochs M, Braun V (1995) Transcriptional regulation of ferric citrate transport in Escherichia coli K-12. Fecl belongs to a new subfamily of sigma 70-type factors that respond to extracytoplasmic stimuli. Mol Microbiol 18(1):163–174. https://doi.org/10.1111/j.1365-2958.1995.mmi_18010163.x
Anthony LC, Foley KM, Thompson NE, Burgess RR (2003) Expression, purification of, and monoclonal antibodies to sigma factors from Escherichia coli. Methods Enzymol 370:181–192. https://doi.org/10.1016/S0076-6879(03)70016-0
Arsène F, Tomoyasu T, Bukau B (2000) The heat shock response of Escherichia coli. Int J Food Microbiol 55(1-3):3–9. https://doi.org/10.1016/s0168-1605(00)00206-3
Battesti A, Majdalani N, Gottesman S (2011) The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol 65:189–213. https://doi.org/10.1146/annurev-micro-090110-102946
Becker G, Klauck E, Hengge-Aronis R (1999) Regulation of RpoS proteolysis in Escherichia coli: the response regulator RssB is a recognition factor that interacts with the turnover element in RpoS. Proc Natl Acad Sci U S A 96(11):6439–6444. https://doi.org/10.1073/pnas.96.11.6439
Brown DR, Barton G, Pan Z, Buck M, Wigneshweraraj S (2014) Nitrogen stress response and stringent response are coupled in Escherichia coli. Nat Commun 5:4115. https://doi.org/10.1038/ncomms5115
Davis MC, Kesthely CA, Franklin EA, MacLellan SR (2017) The essential activities of the bacterial sigma factor. Can J Microbiol 63(2):89–99. https://doi.org/10.1139/cjm-2016-0576
Dylewski M, Fernández-Coll L, Bruhn-Olszewska B, Balsalobre C, Potrykus K (2019) Autoregulation of greA expression relies on GraL rather than on greA promoter region. Int J Mol Sci 20(20):5224. https://doi.org/10.3390/ijms20205224
Enz S, Mahren S, Menzel C, Braun V (2003) Analysis of the ferric citrate transport gene promoter of Escherichia coli. J Bacteriol 185(7):2387–2391. https://doi.org/10.1128/JB.185.7.2387-2391.2003
Enz S, Mahren S, Stroeher UH, Braun V (2000) Surface signaling in ferric citrate transport gene induction: interaction of the FecA, FecR, and FecI regulatory proteins. J Bacteriol 182(3):637–646. https://doi.org/10.1128/JB.182.3.637-646.2000
Feklístov A, Sharon BD, Darst SA, Gross CA (2014) Bacterial sigma factors: a historical, structural, and genomic perspective. Annu Rev Microbiol 68:357–376. https://doi.org/10.1146/annurev-micro-092412-155737
Gottesman S (2019) Trouble is coming: signaling pathways that regulate general stress responses in bacteria. J Biol Chem 294(31):11685–11700. https://doi.org/10.1074/jbc.REV119.005593
Guisbert E, Herman C, Lu CZ, Gross CA (2004) A chaperone network controls the heat shock response in E. coli. Genes Dev 18(22):2812–2821. https://doi.org/10.1101/gad.1219204
Helmann JD (2019) Where to begin? Sigma factors and the selectivity of transcription initiation in bacteria. Mol Microbiol 112(2):335–347. https://doi.org/10.1111/mmi.14309
Lawrence AM, Besir HU (2009) Staining of proteins in gels with Coomassie G-250 without organic solvent and acetic acid. J Vis Exp 30:1350. https://doi.org/10.3791/1350
Liberek K, Galitski TP, Zylicz M, Georgopoulos C (1992) The DnaK chaperone modulates the heat shock response of Escherichia coli by binding to the sigma 32 transcription factor. Proc Natl Acad Sci U S A 89(8):3516–3520. https://doi.org/10.1073/pnas.89.8.3516
Lu Q, Chen T, Wang J, Wang F, Ye W, Ma L, Wu S (2023) Structural insight into the mechanism of σ32-mediated transcription initiation of bacterial RNA polymerase. Biomolecules 13(5):738. https://doi.org/10.3390/biom13050738
Maciag A, Peano C, Pietrelli A, Egli T, De Bellis G, Landini P (2011) In vitro transcription profiling of the σS subunit of bacterial RNA polymerase: re-definition of the σS regulon and identification of σS-specific promoter sequence elements. Nucleic Acids Res 39(13):5338–5355. https://doi.org/10.1093/nar/gkr129
Maeda H, Jishage M, Nomura T, Fujita N, Ishihama A (2000) Two extracytoplasmic function sigma subunits, sigma(E) and sigma(FecI), of Escherichia coli: promoter selectivity and intracellular levels. J Bacteriol 182(4):1181–1184. https://doi.org/10.1128/JB.182.4.1181-1184.2000
Mahren S, Enz S, Braun V (2002) Functional interaction of region 4 of the extracytoplasmic function sigma factor FecI with the cytoplasmic portion of the FecR transmembrane protein of the Escherichia coli ferric citrate transport system. J Bacteriol 184(13):3704–3711. https://doi.org/10.1128/JB.184.13.3704-3711.2002
Marblestone JG, Edavettal SC, Lim Y, Lim P, Zuo X, Butt TR (2006) Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci 15(1):182–189. https://doi.org/10.1110/ps.051812706
Murakami KS, Darst SA (2003) Bacterial RNA polymerases: the wholo story. Curr Opin Struct Biol 13(1):31–39. https://doi.org/10.1016/s0959-440x(02)00005-2
Murakami KS (2015) (2015) Structural biology of bacterial RNA polymerase. Biomolecules 5(2):848–864. https://doi.org/10.3390/biom5020848
Nonaka G, Blankschien M, Herman C, Gross CA, Rhodius VA (2006) Regulon and promoter analysis of the E. coli heat-shock factor, sigma32, reveals a multifaceted cellular response to heat stress. Genes Dev 20(13):1776–1789. https://doi.org/10.1101/gad.1428206
Obrist M, Langklotz S, Milek S, Führer F, Narberhaus F (2009) Region C of the Escherichia coli heat shock sigma factor RpoH (sigma 32) contains a turnover element for proteolysis by the FtsH protease. FEMS Microbiol Lett 290(2):199–208. https://doi.org/10.1111/j.1574-6968.2008.01423.x
Ochs M, Veitinger S, Kim I, Welz D, Angerer A, Braun V (1995) Regulation of citrate-dependent iron transport of Escherichia coli: fecR is required for transcription activation by FecI. Mol Microbiol 15(1):119–132. https://doi.org/10.1111/j.1365-2958.1995.tb02226.x
Österberg S, del Peso-Santos T (2011) Shingler V (2011) Regulation of alternative sigma factor use. Annu Rev Microbiol 65:37–55. https://doi.org/10.1146/annurev.micro.112408.134219
Paget MS (2015) Bacterial sigma factors and anti-sigma factors: structure, function and distribution. Biomolecules 5(3):1245–1265. https://doi.org/10.3390/biom5031245
Panavas T, Sanders C, Butt TR (2009) SUMO fusion technology for enhanced protein production in prokaryotic and eukaryotic expression systems. Methods Mol Biol 497:303–317. https://doi.org/10.1007/978-1-59745-566-4_20
Park K, Choi S, Ko M, Park C (2001) Novel sigmaF-dependent genes of Escherichia coli found using a specified promoter consensus. FEMS Microbiol Lett 202(2):243–250. https://doi.org/10.1111/j.1574-6968.2001.tb10811.x
Peroutka Iii RJ, Orcutt SJ, Strickler JE, Butt TR (2011) SUMO fusion technology for enhanced protein expression and purification in prokaryotes and eukaryotes. Methods Mol Biol 705:15–30. https://doi.org/10.1007/978-1-61737-967-3_2
Potrykus K, Murphy H, Chen X, Epstein JA, Cashel M (2010) Imprecise transcription termination within Escherichia coli greA leader gives rise to an array of short transcripts, GraL. Nucleic Acids Res 38(5):1636–1651. https://doi.org/10.1093/nar/gkp1150
Raina S, Missiakas D, Georgopoulos C (1995) The rpoE gene encoding the sigma E (sigma 24) heat shock sigma factor of Escherichia coli. EMBO J 14(5):1043–1055. https://doi.org/10.1002/j.1460-2075.1995.tb07085.x
Rial DV, Ceccarelli EA (2002) Removal of DnaK contamination during fusion protein purifications. Protein Expr Purif 25(3):503–507. https://doi.org/10.1016/s1046-5928(02)00024-4
Rouvière PE, De Las PA, Mecsas J, Lu CZ, Rudd KE, Gross CA (1995) rpoE, the gene encoding the second heat-shock sigma factor, sigma E, in Escherichia coli. EMBO J 14(5):1032–1042. https://doi.org/10.1002/j.1460-2075.1995.tb07084.x
Shikalov AB, Esyunina DM, Pupov DV, Kulbachinskiy AV, Petushkov IV (2019) The σ24 Subunit of Escherichia coli RNA polymerase can induce transcriptional pausing in vitro. Biochemistry (Mosc) 84(4):426–434. https://doi.org/10.1134/S0006297919040102
Shimada T, Tanaka K, Ishihama A (2017) The whole set of the constitutive promoters recognized by four minor sigma subunits of Escherichia coli RNA polymerase. PLoS One 12(6):e0179181. https://doi.org/10.1371/journal.pone.0179181
Shingler V (2011) Signal sensory systems that impact σ54-dependent transcription. FEMS Microbiol Rev 35(3):425–440. https://doi.org/10.1111/j.1574-6976.2010.00255.x
Sobala M, Bruhn-Olszewska B, Cashel M, Potrykus K (2019) Methylobacterium extorquens RSH enzyme synthesizes (p)ppGpp and pppApp in vitro and in vivo, and leads to discovery of pppApp synthesis in Escherichia coli. Front Microbiol 10:859. https://doi.org/10.3389/fmicb.2019.00859
Van Hove B, Staudenmaier H, Braun V (1990) Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J Bacteriol 172(12):6749–6758. https://doi.org/10.1128/jb.172.12.6749-6758.1990
Wade JT, Castro Roa D, Grainger DC, Hurd D, Busby SJ, Struhl K, Nudler E (2006) Extensive functional overlap between sigma factors in Escherichia coli. Nat Struct Mol Biol 13(9):806–814. https://doi.org/10.1038/nsmb1130
Acknowledgements
We would like to acknowledge Karolina Balcer who carried out construction of the pKB1 plasmid that was used as the cloning vector in this study.
Funding
Funding for this work was provided by the National Science Centre (Poland) to K.P. (grant number UMO-2021/43/B/NZ2/00855).
Author information
Authors and Affiliations
Contributions
Study conception, supervision, funding acquisition, and first draft writing by K.P. Methodology and experiments were designed by K.P. and B.K. All authors contributed to material preparation, data collection and analysis. All authors commented on the manuscript, have read, and approved its final version.
Corresponding author
Ethics declarations
Ethics approval
This work did not involve studies of human or animal subjects.
Consent to participate
Not applicable.
Consent for publication
All authors agreed on the publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by: Agnieszka Szalewska-Palasz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 873 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kędzierska, B., Stodolna, A., Bryszkowska, K. et al. A simple and unified protocol to purify all seven Escherichia coli RNA polymerase sigma factors. J Appl Genetics (2024). https://doi.org/10.1007/s13353-024-00870-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13353-024-00870-3