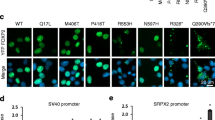
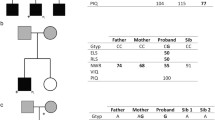
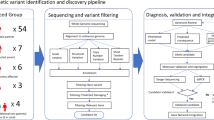
Abstract
Since its initial identification, the Forkhead Box P2 gene (FOXP2) has maintained its singular status as the archetypal monogenic determinant implicated in Mendelian forms of human speech and language impairments. Despite the passage of two decades subsequent to its discovery, extant literature remains disproportionately sparse with regard to case-specific instances and loci of mutational perturbations. The objective of the current investigation centers on furnishing an enriched delineation of both its clinical manifestations and its mutational heterogeneity. Clinical phenotypes and peripheral blood samples were assiduously amassed from familial subjects. Whole-exome sequencing and Sanger sequencing methodologies were deployed for the unambiguous identification of potential genetic variants and for corroborating their co-segregation within the family pedigree. An exhaustive review of published literature focusing on patients manifesting speech and language disorders consequent to FOXP2 genetic anomalies was also undertaken. The investigation yielded the identification of a novel heterozygous variant, c.661del (p.L221Ffs*41), localized within the FOXP2 gene in the proband, an inheritance from his symptomatic mother. The proband presented with an array of symptoms, encompassing dysarthric speech, deficits in instruction comprehension, and communicative impediments. In comparison, the mother exhibited attenuated symptoms, including rudimentary verbalization capabilities punctuated by pronounced stuttering and dysarthria. A comprehensive analysis of articles archived in the Human Gene Mutation Database (HGMD) classified under “DM” disclosed the existence of 74 patients inclusive of the subjects under current examination, sub-divided into 19 patients with null variants, 5 patients with missense variants, and 50 patients with gross deletions or complex genomic rearrangements. A conspicuous predominance of delayed speech, impoverished current verbal abilities, verbal comprehension deficits, and learning difficulties were observed in patients harboring null or missense FOXP2 variants, as compared to their counterparts with gross deletions or complex rearrangements. Developmental delays, hypotonia, and craniofacial aberrations were exclusive to the latter cohort. The elucidated findings augment the existing corpus of knowledge on the genetic architecture influencing both the proband and his mother within this specified familial context. Of critical importance, these discoveries furnish a robust molecular framework conducive to the prenatal diagnostic evaluations of prospective progeny within this familial lineage.


Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Change history
25 March 2024
A Correction to this paper has been published: https://doi.org/10.1007/s13353-024-00851-6
Abbreviations
- FOXP2:
-
Forkhead box P2
- ASD:
-
Autism spectrum disorder
- CAS:
-
Childhood apraxia of speech
- SLI:
-
Speech and language impairments
- DVD:
-
Developmental verbal dyskinesia
- aCGH:
-
Array comparative genomic hybridization
- MLPA:
-
Multiplex ligation-dependent probe amplification
References
Campbell P, Reep RL, Stoll ML, Ophir AG, Phelps SM (2009) Conservation and diversity of Foxp2 expression in muroid rodents: functional implications. J Comp Neurol 512:84–100
Carrion-Castillo A, Franke B, Fisher SE (2013) Molecular genetics of dyslexia: an overview. Dyslexia (Chichester, England) 19:214–240
Co M, Anderson AG, Konopka G (2020) FOXP transcription factors in vertebrate brain development, function, and disorders. Wiley Interdiscip Rev Dev Biol 9:e375
d’Hennezel E, Bin Dhuban K, Torgerson T, Piccirillo CA (2012) The immunogenetics of immune dysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet 49:291–302
Enard W, Przeworski M, Fisher SE, Lai CS, Wiebe V, Kitano T, Monaco AP, Pääbo S (2002) Molecular evolution of FOXP2, a gene involved in speech and language. Nature 418:869–872
Ferland RJ, Cherry TJ, Preware PO, Morrisey EE, Walsh CA (2003) Characterization of Foxp2 and Foxp1 mRNA and protein in the developing and mature brain. J Comp Neurol 460:266–279
Le Fevre AK, Taylor S, Malek NH, Horn D, Carr CW, Abdul-Rahman OA, O'Donnell S, Burgess T, Shaw M, Gecz J, Bain N, Fagan K, Hunter MF (2013) FOXP1 mutations cause intellectual disability and a recognizable phenotype. Am J Med Genet A 161a:3166–3175
Fisher SE, Scharff C (2009) FOXP2 as a molecular window into speech and language. Trends in Genetics: TIG 25:166–177
Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C (2004) FoxP2 expression in avian vocal learners and non-learners. J Neurosci: Off J Soc Neurosci 24:3164–3175
Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP (2001) A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413:519–523
Lai CS, Gerrelli D, Monaco AP, Fisher SE, Copp AJ (2003) FOXP2 expression during brain development coincides with adult sites of pathology in a severe speech and language disorder. Brain: a J. Neuro 126:2455-2462
MacDermot KD, Bonora E, Sykes N, Coupe AM, Lai CS, Vernes SC, Vargha-Khadem F, McKenzie F, Smith RL, Monaco AP, Fisher SE (2005) Identification of FOXP2 truncation as a novel cause of developmental speech and language deficits. Am J Hum Genet 76:1074–1080
Morgan A, Fisher SE, Scheffer I, Hildebrand M. (1993). FOXP2-related speech and language disorder. In: GeneReviews(®), M.P. Adam, J. Feldman, G.M. Mirzaa, R.A. Pagon, S.E. Wallace, L.J.H. Bean, K.W. Gripp, andA. Amemiya, eds. (Seattle (WA), University of Washington, Seattle. Copyright © 1993–2023, University of Washington, Seattle. GeneReviews is a registered trademark of the University of Washington, Seattle
Newbury DF, Monaco AP (2010) Genetic advances in the study of speech and language disorders. Neuron 68:309–320
Newbury DF, Mari F, Sadighi Akha E, Macdermot KD, Canitano R, Monaco AP, Taylor JC, Renieri A, Fisher SE, Knight SJ (2013) Dual copy number variants involving 16p11 and 6q22 in a case of childhood apraxia of speech and pervasive developmental disorder. Eur J Hum Genet: EJHG 21:361–365
Reader RH, Covill LE, Nudel R, Newbury DF (2014) Genome-wide studies of specific language impairment. Curr Behav Neurosci Rep 1:242–250
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med: Off J Am College Med Genet 17:405–424
Shao J, Diamond MI (2007) Polyglutamine diseases: emerging concepts in pathogenesis and therapy. Hum Mol Genet 16(2):R115-123
Shu W, Yang H, Zhang L, Lu MM, Morrisey EE (2001) Characterization of a new subfamily of winged-helix/forkhead (Fox) genes that are expressed in the lung and act as transcriptional repressors. J Biol Chem 276:27488–27497
Takahashi K, Liu FC, Hirokawa K, Takahashi H (2003) Expression of Foxp2, a gene involved in speech and language, in the developing and adult striatum. J Neurosci Res 73:61–72
Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA (2004) Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J Neurosci: Off J Soc Neurosci 24:3152–3163
Thevenon J, Callier P, Andrieux J, Delobel B, David A, Sukno S, Minot D, Mosca Anne L, Marle N, Sanlaville D, Bonnet M, Masurel-Paulet A, Levy F, Gaunt L, Farrell S, Le Caignec C, Toutain A, Carmignac V, Mugneret F, Clayton-Smith J, Thauvin-Robinet C, Faivre L (2013) 12p13.33 microdeletion including ELKS/ERC1, a new locus associated with childhood apraxia of speech. Eur J Hum Genet: EJHG 21:82–88
Tønne E, Due-Tønnessen BJ, Vigeland MD, Amundsen SS, Ribarska T, Åsten PM, Sheng Y, Helseth E, Gilfillan GD, Mero IL, Heimdal KR (2022) Whole-exome sequencing in syndromic craniosynostosis increases diagnostic yield and identifies candidate genes in osteogenic signaling pathways. Am J Med Genet A 188:1464–1475
Turner SJ, Hildebrand MS, Block S, Damiano J, Fahey M, Reilly S, Bahlo M, Scheffer IE, Morgan AT (2013) Small intragenic deletion in FOXP2 associated with childhood apraxia of speech and dysarthria. Am J Med Genet A 161a: 2321–2326
Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38:e164
Acknowledgements
We would like to thank all the participating family members in this study and Berry Genomics Co. for their technical support.
Funding
This work was supported by Xi’an Children’s Hospital research project (2021D01) and the Generic Technology Research platform of Shaanxi province 2023GXJS-01.
Author information
Authors and Affiliations
Contributions
FC and YY contributed to the conception and design of the study. LW collected the clinical data. CL wrote the first draft of the manuscript. HW and QC prepared Figs. 1 and 2. LZ, LM, and LB coordinated and supervised data collection. All authors contributed to the manuscript revision and read and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Ethics Committee of Xi’an Children’s Hospital. The article does not contain any personally identifiable information, and informed consent had been obtained.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Ewa Ziętkiewicz.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The original article contains an error. The authorship under the article note should be change from: “Fengyu Che, Chenhao Li, Lifang Wang, and Ying Yang contributed equally to this work.” to: “Fengyu Che and Chenhao Li contributed equally to this work”
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Che, F., Li, C., Zhang, L. et al. Novel FOXP2 variant associated with speech and language dysfunction in a Chinese family and literature review. J Appl Genetics 65, 367–373 (2024). https://doi.org/10.1007/s13353-024-00849-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13353-024-00849-0