Abstract
The aim of this study was to evaluate the participation of polymorphism at position C421A and mRNA expression of the ABCG2 gene in the development of peptic ulcers, which is a very common and severe disease. ABCG2, encoded by the ABCG2 gene, has been found inter alia in the gastrointestinal tract, where it plays a protective role eliminating xenobiotics from cells into the extracellular environment. The materials for the study were biopsies of gastric mucosa taken during a routine endoscopy. For genotyping by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) at position C421A, DNA was isolated from 201 samples, while for the mRNA expression level by real-time PCR, RNA was isolated from 60 patients. The control group of healthy individuals consisted of 97 blood donors. The dominant genotype in the group of peptic ulcer patients and healthy individuals was homozygous CC. No statistically significant differences between healthy individuals and the whole group of peptic ulcer patients and, likewise, between the subgroups of peptic ulcer patients (infected and uninfected with Helicobacter pylori) were found. ABCG2 expression relative to GAPDH expression was found in 38 of the 60 gastric mucosa samples. The expression level of the gene varies greatly among cases. The statistically significant differences between the intensity (p = 0.0375) of H. pylori infection and ABCG2 gene expression have been shown. It was observed that the more intense the infection, the higher the level of ABCG2 expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peptic ulcer (PU) is a disease with a high percentage of morbidity. Due to its complications such as bleeding, pyloric stenosis and life-threatening perforations occurring in 10–20 % of patients, peptic ulcer diseases are a serious condition. According to the Demographic Yearbook of Poland, in 2012, 1886 people died because of duodenal and gastric ulcer in Poland (Dmochowska 2014; Malfertheiner et al. 2009; Ramakrishnan and Salinas 2007; Szyca et al. 2012; Thorsen et al. 2013).
The development of peptic ulcer may be influenced not only by environmental factors, e.g. cigarette smoking, alcohol consumption, diet high in salt, steroids and non-steroidal anti-inflammatory drugs, Helicobacter pylori infection and stress, but also a patient’s genetic predisposition (Schabowski 2002; Thorsen et al. 2013). It concerns changes in the genes physiologically involved in the protection of gastric mucosa.
One of the plausible genetic factors contributing to the development of peptic ulcer disease could be changes in the ABCG2 gene. It encodes protein named ABCG2 with 655 amino acids (previously BCRP, breast cancer resistance protein), which is a half-transporter belonging to the ABC transporters superfamily. As for all proteins in this family, ABCG2 uses energy from ATP hydrolysis to transport substrates. The protein has been found inter alia in the brain, blood–brain barrier, prostate, ovaries, testes, placenta, adrenal gland and gastrointestinal tract, where it plays a protective role eliminating xenobiotics from cells into the extracellular environment. In the digestive tract, ABCG2 is localised in the apical membrane of cells, which confirms the hypothesis of its protective role by limiting accumulation in cells/organs of harmful xenobiotics. On the other hand, one loss of its function could promote peptic ulcer development (Mao and Unadkat 2005; Mo and Zhang 2012; Liu and Liu 2013).
The ABCG2 gene is highly polymorphic. So far, over 40 single nucleotide polymorphisms (SNPs) have been found (Iida et al. 2002). SNPs may affect the level of mRNA expression, which leads to a change/loss of ABCG2 function. This could result in the accumulation of xenobiotics in cells and, thus, in the increased risk of peptic ulcer disease development. One of such polymorphisms is the SNP substitution of C to T at position 421. It is a non-synonymous polymorphism leading to a change of glutamine into lysine at position 141 in protein (Q141K) (Cusatis et al. 2006). The ABCG2 gene polymorphism at position 421 is associated with a lower expression and a lower transport activity of encoded protein (Imai et al. 2002; Kondo et al. 2004; Mizuarai et al. 2004).
Fehér et al. (2013) found a significantly increased susceptibility to Alzheimer’s disease [odds ratio (OR) = 1.741, 95 % confidence interval (CI): 1.075–2.819, p = 0.024) associated with the ABCG2 C/C genotype when compared with the variant allele containing genotypes CA and AA as the control group. In pancreatic cancer, such a correlation was not found (Pang et al. 2014).
The aim of this study was to evaluate the participation of polymorphism at position C421A and the mRNA expression level of the ABCG2 gene in the development of peptic ulcer. To the best of our knowledge, this area has not been explored so far.
Materials and methods
The materials for the study were biopsies of gastric mucosa taken during endoscopy from patients with peptic ulcer diagnosed at the Department of Surgery, District Hospital, Leczyca, Poland. The obtained samples were residual material remaining after a routine diagnostics.
A small amount of biopsies material did not allow the simultaneous isolation of two nucleic acids (DNA and RNA) from one sample. Therefore, in order to analyse both polymorphism at position C421A and mRNA expression, the group of patients with peptic ulcer disease diagnosed at the same time in the Department of Surgery, District Hospital, Leczyca, Poland was divided into two investigated groups. The first group consisted of 201 patients (129 females; 72 males; median age of the group: 53 years), from whom DNA was isolated in order to genotype C421A of ABCG2 (investigated group I). The other group consisted of 60 patients (34 females; 26 males; median age of the group: 60 years), from whom RNA was isolated in order to assess the mRNA expression level (investigated group II). Patients who were treated with non-steroidal anti-inflammatory drugs were excluded from the study.
The group of healthy individuals consisted of 97 blood donors (58 females; 39 males; median age of the group: 33 years) from the local blood bank, and they geographically and ethnically matched the group of patients with peptic ulcer.
Data concerning exposure to carcinogens in patients and healthy individuals were not available. The investigation was in accordance with the principles of the Declaration of Helsinki, and was approved by the Ethical Committee of the Medical University of Lodz (RNN/285/13/KE; RNN/195/13/KE). All individuals included in the study gave informed consent.
DNA and RNA isolation
DNA and RNA from biopsies of gastric mucosa collected during a routine endoscopy were isolated according to the Genomic DNA Mini and Total RNA Mini protocols, respectively (A&A Biotechnology, Poland). The purity and concentration of DNA and RNA samples were assessed spectrophotometrically. Until analysis, DNA and RNA samples had been stored at −20 °C and −76 °C, respectively.
Genotyping of C421A
PCR
For the studied polymorphism, polymerase chain reaction (PCR) was performed in accordance with the AccuTaq™ LA DNA Polymerase Kit protocol (Sigma Aldrich, Germany). The mixture for PCR consisted of 10× AccuTaq buffer, 1.5 mM of MgCl2, 0.5 μM of each primer (C421A F 5′-ATGTTGTGATGGGCACTCTG-3′; C421A R 5′-TGCTGATCATGATGCTTTCAG-3′), 0.2 mM of each dNTP, 0.5U of AccuTaq LA DNA Polymerase, 50 ng of DNA template and distilled water to a final volume of 20 μL. In every experiment, a negative control was included. Products of the PCR reactions were assessed by electrophoresis in 2 % agarose gel. Reaction products for the investigated SNP had a size of 184 bp.
RFLP
Genotyping of C421A was performed by restriction fragment length polymorphism (RFLP). Amplified DNA fragments for the SNP on position C421A were digested by MseI (New England Biolabs, USA) for 16 h at 30 °C. Genotypes were identified by electrophoresis of amplified DNA fragments after digestion by restriction enzyme (two bands of 84 and 100 bp for genotype CC; three bands of 36, 64 and 84 bp for genotype AA; four bands of 36, 64, 84 and 100 bp for genotype CA).
Expression of ABCG2 mRNA
Reverse transcription
A total cellular RNA was transcribed into complementary DNA (cDNA) in accordance with the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA). The final concentration of RNA in the reaction mixture was 0.01 μg/μL. Synthesised cDNA was stored at −20 °C until analysis. As a reference gene, GAPDH was used. Only the samples which showed the presence of PCR product for the GAPDH gene were included in the analysis.
PCR
For qualitative analysis of the mRNA expression of the reference gene GAPDH, a PCR assay was performed in accordance with the AccuTaq™ LA DNA Polymerase Kit protocol (Sigma Aldrich, Germany). The mixture for PCR consisted of 10× AccuTaq buffer, 1 mM of MgCl2, 0.5 μM of each primer (GAPDH gene: F 5′-TGGTATCGTGGAAGGACTCATGAC-3′, R 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′), 0.2 mM of each dNTP, 0.5U of AccuTaq LA DNA Polymerase, 0.2 μg of cDNA template and distilled water to a final volume of 20 μL. In every experiment, a negative control was included. Products of the PCR reactions were assessed by electrophoresis in 2 % agarose gel. The reaction product for GAPDH had a size of 257 bp.
Real-time PCR
Quantification assessment of ABCG2 (investigated gene) and GAPDH (reference gene) mRNA was performed by real-time PCR using a Rotor-Gene™ 6000 (Corbett Research, Germany), according to the KAPA SYBR® FAST qPCR Kit Master Mix (2X) Universal protocol (Kapa Biosystems, USA). The reaction mixture for both genes consisted of 10 μL KAPA SYBR FAST qPCR Master Mix (2X), 0.4 μL of each primer (ABCG2 gene: F 5′-ATGTCAACTCCTCCTTCTAC-3′; R 5′-AATGATCTGAGCTATAGAGGC-3′; GAPDH gene: F 5′-TGGTATCGTGGAAGGACTCATGAC-3′, R 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′), 1.5 μL of cDNA and distilled water up to a final volume of 20 μL. The reactions for ABCG2 and GAPDH were carried out in separate tubes. Samples were tested in triplicate, and the mean of the obtained Ct values for both ABCG2 and GAPDH was calculated. In each experiment, a negative control, also tested in triplicate, was included. For estimation of the kinetic PCR reaction efficiency, the standard curves for both genes, by four serial 10-fold dilutions of a quantified PCR product (obtained with the same primers as those used in the quantification mRNA analysis), were constructed. The efficiencies were calculated from the slopes of the standard curves according to the equation E = 10[−1/slope] − 1. Because the efficiencies for both genes were different (ABCG2 122 %; GAPDH 107 %), the Pfaffl method was used to calculate relative changes in gene expression (Livak and Schmittgen 2001; Tyburski et al. 2008). The mean Ct values for the ABCG2 and GAPDH genes for all investigated samples were utilised as calibrators.
Statistical analysis
All statistical analyses were performed using STATISTICA 10 (StatSoft, Inc., 2011). The χ2 test, χ2 test with Yates’ correction and V2 test were applied to evaluate conformity between the observed and expected genotype frequencies according to the Hardy–Weinberg rule and to determine the significance of differences in allele and genotype frequencies between patients and controls and between subgroups of patients. The collected quantitative data were tested to check for conformity with a normal distribution on the basis of the Shapiro–Wilk test. Because of the lack of normality of ABCG2 expression values, decimal logarithm of the expression level was used for statistical analysis. Student’s t-test and analysis of variance (ANOVA) were applied to assess the differences in the mean values of the ABCG2 expression between the subgroups of patients. In all conducted tests, a p-value < 0.05 was assumed to be significant.
Results
Genotyping of ABCG2
All 201 biopsy specimen of gastric mucosa (peptic ulcer patients; investigated group I) and 97 blood samples (healthy individuals) for the SNP at position C421A of the ABCG2 gene were successfully analysed. All genotypes for the investigated polymorphism in both peptic ulcer patients and healthy individuals were in Hardy–Weinberg equilibrium.
Firstly, genotype and allele frequencies for the polymorphism at position C421A between peptic ulcer patients (investigated group I) and healthy individuals were compared (Table 1). For both genotype and allele of the studied SNP, no statistically significant differences between peptic ulcer patients and healthy individuals were found (p = 0.7845 and 0.9370, respectively). The dominant genotype in both groups was homozygous CC (97.5 % for peptic ulcer and 97.9 % for healthy individuals), whereas genotype AA occurred only in the group of patients with peptic ulcer (0.5 %).
Secondly, on the basis of the results of rapid urease tests, the group of peptic ulcer patients were divided into two subgroups: patients infected with H. pylori and patients uninfected with this bacterium. The frequency of genotypes and alleles of the SNP C421A for these two subgroups was compared. No statistically significant differences for the studied polymorphism were observed (p = 0.3591 and 0.2127, respectively). Nevertheless, it is noteworthy that allele A occurred more frequently in the subgroup of patients uninfected with H. pylori than in the infected subgroup (2.5 % and 0.5 %, respectively). The results are shown in Table 2.
In the last step of the analysis, the subgroups of patients infected and uninfected with H. pylori were divided further according to gender. First, a comparison of the incidence of genotype and allele of polymorphism C421A between the subgroup of infected women and a subgroup of women not infected with H. pylori was conducted. No statistically significant differences were found (p = 0.3524 and 0.2127, respectively). However, allele A occurred more frequently in the subgroup of uninfected women than in the subgroup of women infected with H. pylori. This observation was similar to the results obtained in the whole subgroup of patients uninfected with this bacterium. On the other hand, among men, no genotype AA was found. Therefore, no analogous analysis was possible.
Expression of mRNA ABCG2
Initially, a qualitative analysis of the GAPDH gene was performed to check the reverse transcription of the procedure. In all tested samples, the expression of GAPDH was present. ABCG2 expression was found in 38 of the 60 gastric mucosa samples. There were no statistical differences between gender, age and presence of the investigated gene expression (Table 3). There was also no correlation between the presence of H. pylori infection and the presence of gene expression, but in a more intensive infection, ABCG2 expression appeared more frequently (p = 0.0324, Table 3).
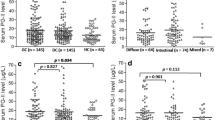
The expression level of ABCG2 relative to GAPDH varies greatly among cases. It ranged from −1.64 to 1.75, with a mean value of −0.13 (standard deviation 0.77). There was no association between ABCG2 expression level and the patient’s gender (p = 0.4957). Also, no connection between the level and patient’s age was stated (p = 0.5329, Table 4). No difference in the expression level was also stated when it was compared between patients <60 and >60 years old. There was no significant connection between the presence of H. pylori infection and the expression level of ABCG2 (p = 0.2864, Table 4). In the H. pylori-infected patients divided into three subgroups according the infection intensity, the expression level of the investigated gene differed significantly between the subgroups (p = 0.0375, Table 4). The more intense the infection, the higher the level of ABCG2 expression was observed. The post-hoc analysis showed that there was a significant difference in the expression level between the subgroups of low and high intensity of infection (p = 0.0306, Fig. 1).
Discussion
ABCG2 was described for the first time in tumour cells, where it produced a significant resistance to chemotherapeutic agents (Doyle et al. 1998). The transporter on cells weakens access of drugs to the cellular target and, thereby, contributes to a reduction in the efficacy of the applied therapy. As ABCG2 has a low substrate specificity, it might contribute to the resistance to structurally diverse anti-cancer drugs, a phenomenon called multidrug resistance (MDR) (Gottesman et al. 2002). However, ABCG2 is present not only in tumour cells, and its function in the development of MDR is a manifestation of the physiological role of this protein.
Protein product of the ABCG2 gene is located in many normal tissues, including the gastrointestinal tract, where it plays a protective role by removing endogenous and exogenous xenobiotics, i.e. bacterial toxins from cells to the extracellular environment. Any changes in the ABCG2 gene, such as SNPs or in gene mRNA expression, may influence the protein level and/or its function. That may lead to a loss of its protective function and, thus, increase the risk of peptic ulcer development. Imai et al. showed that the occurrence of allele A of the SNP at position C421A may be associated with a lower expression of the protein in comparison to wild-type C of this polymorphism. It was suggested that the substitution of glutamine by lysine on position 141, as a consequence of the studied SNP, may lead to the increased susceptibility to degradation of the protein. This is a consequence of the fact that lysine and glutamine have different electronic charges, which might alter the tertiary structure of protein (Imai et al. 2002).
So far, to the best of our knowledge, participation of the ABCG2 gene polymorphism at position C421A and mRNA expression in the peptic ulcer development has not been investigated. All genotypes in the peptic ulcer patients and healthy individuals were in accordance with the Hardy–Weinberg rule, which proves their representativeness of the studied population (Table 1). In this research, the dominant genotype was homozygous CC, which is similar to the results in European Caucasian (de Jong et al. 2004), non-Hispanic Caucasian (Gardner et al. 2008), Hungarian (Fehér et al. 2013) and Sub-African populations (de Jong et al. 2004), but which was different in Han Chinese (Hu et al. 2007), Korean (Kim et al. 2010) and Japan (Imai et al. 2002) populations. Contrary to our results, the incidence of genotype CC in those studies was lower but homozygous AA occurred with a greater frequency than in Caucasian and African populations.
Through the comparison of genotyping results between peptic ulcer patients and healthy individuals, the impact of the investigated polymorphism on peptic ulcer development was estimated. No statistically significant differences for this polymorphism were found (Table 1). Therefore, it can be assumed that the studied polymorphism is not associated with an increased risk of developing peptic ulcer disease. However, the results may confirm a protective role of ABCG2, which may be associated with the presence of the CC genotype of the SNP on position C421A. This genotype is connected with a higher expression and transport activity of protein (Morisaki et al. 2005), which, in turn, will lead to a greater elimination of toxins/xenobiotics from gastric mucosa cells. Sparreboom et al. (2005), in a study on the cell line HEK293, showed that allele C of C421A was responsible for the overexpression of ABCG2 and decreased cell accumulation of topotecan. On the other hand, HEK293 cells with the allele A variant of the studied SNP showed an increase in the intracellular accumulation of topotecan.
Our hypothesis may be confirmed by the results of the research presented by Gardner at el. (2008), who also demonstrated no statistical significance between polymorphism at position C421A and risk of prostate cancer development, but a higher incidence of the CC genotype was associated with increased survival in patients with this cancer. Zhou et al. (2014) showed that the occurrence of genotype AA and allele A was associated with higher risk of gout development in the male Han Chinese population.
This is the first report concerning ABCG2 expression in the gastric mucosa of peptic ulcer patients. We found expression of the gene in 63.3 % of analysed samples. Previously, ABCG2 protein was investigated by immunohistochemistry in selected human tissues. Prominent ABCG2 immunostaining was stated in the gastrointestinal tract, with strong apical staining of the epithelium of the small intestine and colon, but no staining in the stomach epithelium was found. ABCG2/PBGD mRNA expression ratios in this tissue measured by qualitative RT-PCR was relatively low (Maliepaard et al. 2001). In the presented research, in samples positive for ABCG2 expression, the level of expression differed highly among cases. As ABCG2 protein was not stated by others (Maliepaard et al. 2001) in the epithelium of the stomach, the observed expression of ABCG2 could derive from the capillaries endothelial cells, where expression of the protein was shown (Diestra et al. 2002; Maliepaard et al. 2001).
ABCG2 expression is regulated by hormones. The ABCG2 promoter estrogen and progesterone response elements were described. It has been shown (Ee et al. 2004; Mao 2008) that these hormones increase the level of mRNA or BCRP, which could suggest that some gender-dependent differences in the ABCG2 expression level exist. However, we found no association between gender and the presence of ABCG2 expression. Similarly, no connection between gender and level of the ABCG2 expression was stated. It remains in agreement with research results obtained by Gutmann et al. (2005), who showed that the expression of ABCG2 mRNA was not significantly different between males and females, in neither the duodenum and the terminal ileum nor in the different colonic segments of the human gastrointestinal tract.
Recently, we have shown that the expression level of another gene belonging to the ABC transporter superfamily, namely ABCB1, is associated with age in peptic ulcer patients (Jażdżyk et al. 2014). ABCB1 expression was higher in older patients. There is also some evidence that ABCG2 could exhibit a differential level of the expression depending on age. Kawase et al. (2015) stated that the mRNA level of ABCG2 decreased with age in the liver of female rats (but not in males). On the other hand, when the expression of ABCG2 by liquid chromatography coupled with tandem mass spectrometry in the human liver was investigated, the expression was not associated with age, gender or mRNA expression (Prasad et al. 2013). In the presented study, neither the presence nor the level of the expression of ABCG2 were connected with age and gender. Considering this and the previously published contradictory findings, the existence of a link between ABCG2 expression and age and gender remains unanswered.
Petrovic et al. (2015) examined the effect of chorioamnionitis, a bacterial intra-amniotic infection, on the expression of the ABCG2 gene and related protein in human placenta. Expression of both the gene and the protein was downregulated in placentas from women with infection relative to healthy controls. In our study, the impact of H. pylori infection on mRNA expression of the transporter in gastric mucosa was analysed. Neither the presence nor the level of expression of ABCG2 were connected with the presence of H. pylori infection in the investigated peptic ulcer patients. However, when the subgroup of infected patients was analysed, the presence of expression was stated to be significantly more frequent in patients of medium or high intensity of H. pylori infection in relation to those of low intensity of infection. Moreover, it was discovered that the more intense the infection, the higher the mean value of the expression. The obtained results could suggest that H. pylori could potentiate the expression of the investigated gene. In the above-mentioned research, Petrovic et al. (2015) showed that, in the placenta of infected woman, the ABCB1 expression level correlates with the expression level of interleukin-6 (IL-6), interleukin-1β (IL-1β) and tumour necrosis factor-α (TNF-α), which could indicate the involvement of these cytokines in expression regulation of the gene. Evseenko et al. (2007) showed that the treatment of primary term trophoblasts with TNF-α and IL-1β significantly decreased ABCG2 protein and mRNA expression, but IL-6 had no significant effect. On the other hand, epidermal growth factor (EGF) and insulin-like growth factor II significantly increased ABCG2 protein and mRNA expression. It could also be speculated that ABCG2 expression in the mucosa of peptic ulcer patients could be under an influence of inflammatory cytokine. However, it should be confirmed using a histopathological assessment of H. pylori density and inflammation degree in a larger group of patients.
The aim of this study was to evaluate the participation of polymorphism at position C421A and mRNA expression of the ABCG2 gene in the development of peptic ulcer.
Conclusion
Based on these studies, it can be concluded that the ABCG2 gene has no connection with the development of peptic ulcers, although Helicobacter pylori infection may lead to increased expression levels of ABCG2 mRNA, because, with the more intense infection with bacterium, a higher level of ABCG2 expression was observed.
On the other hand, in this study, because of the small amounts of biopsies material studied, patients with peptic ulcer were divided into two investigated groups and the analyses of C421A single nucleotide polymorphism (SNP) and mRNA expression of ABCG2 were performed independently of each other. However, as the literature suggests, ABCG2 expression is largely determined by genetic polymorphisms; thus, it would be beneficial to compare, in the future, the results of genotyping and expression data in the same group of patients with peptic ulcer.
References
Cusatis G, Gregorc V, Li J, Spreafico A, Ingersoll RG, Verweij J, Ludovini V, Villa E, Hidalgo M, Sparreboom A, Baker SD (2006) Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst 98(23):1739–1742
de Jong FA, Marsh S, Mathijssen RHJ, King C, Verweij J, Sparreboom A, McLeod HL (2004) ABCG2 pharmacogenetics: ethnic differences in allele frequency and assessment of influence on irinotecan disposition. Clin Cancer Res 10(17):5889–5894
Diestra JE, Scheffer GL, Català I, Maliepaard M, Schellens JH, Scheper RJ, Germà-Lluch JR, Izquierdo MA (2002) Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol 198(2):213–219
Dmochowska H (ed) (2014) Demographic yearbook of Poland. Statistical Publishing Establishment, Warsaw
Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 95(26):15665–15670
Ee PL, Kamalakaran S, Tonetti D, He X, Ross DD, Beck WT (2004) Identification of a novel estrogen response element in the breast cancer resistance protein (ABCG2) gene. Cancer Res 64(4):1247–1251
Evseenko DA, Paxton JW, Keelan JA (2007) Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos 35(4):595–601
Fehér Á, Juhász A, László A, Pákáski M, Kálmán J, Janka Z (2013) Association between the ABCG2 C421A polymorphism and Alzheimer’s disease. Neurosci Lett 550:51–54
Gardner ER, Ahlers CM, Shukla S, Sissung TM, Ockers SB, Price DK, Hamada A, Robey RW, Steinberg SM, Ambudkar SV, Dahut WL, Figg WD (2008) Association of the ABCG2 C421A polymorphism with prostate cancer risk and survival. BJU Int 102(11):1694–1699
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2(1):48–58
Gutmann H, Hruz P, Zimmermann C, Beglinger C, Drewe J (2005) Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem Pharmacol 70(5):695–699
Hu LL, Wang XX, Chen X, Chang J, Li C, Zhang Y, Yang J, Jiang W, Zhuang SM (2007) BCRP gene polymorphisms are associated with susceptibility and survival of diffuse large B-cell lymphoma. Carcinogenesis 28(8):1740–1744
Iida A, Saito S, Sekine A, Mishima C, Kitamura Y, Kondo K, Harigae S, Osawa S, Nakamura Y (2002) Catalog of 605 single-nucleotide polymorphisms (SNPs) among 13 genes encoding human ATP-binding cassette transporters: ABCA4, ABCA7, ABCA8, ABCD1, ABCD3, ABCD4, ABCE1, ABCF1, ABCG1, ABCG2, ABCG4, ABCG5, and ABCG8. J Hum Genet 47:285–310
Imai Y, Nakane M, Kage K, Tsukahara S, Ishikawa E, Tsuruo T, Miki Y, Sugimoto Y (2002) C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther 1:611–616
Jażdżyk M, Sałagacka A, Zebrowska M, Balcerczak M, Mirowski M, Balcerczak E (2014) ABCB1 expression in peptic ulcer patients and its connection with H. pylori Infection. Ann Clin Lab Sci 44(3):294–297
Kawase A, Ito A, Yamada A, Iwaki M (2015) Age-related changes in mRNA levels of hepatic transporters, cytochrome P450 and UDP-glucuronosyltransferase in female rats. Eur J Drug Metab Pharmacokinet 40(2):239–244. doi:10.1007/s13318-014-0208-7
Kim KA, Joo HJ, Park JY (2010) ABCG2 polymorphisms, 34G>A and 421C>A in a Korean population: analysis and a comprehensive comparison with other populations. J Clin Pharm Ther 35(6):705–712
Kondo C, Suzuki H, Itoda M, Ozawa S, Sawada J, Kobayashi D, Ieiri I, Mine K, Ohtsubo K, Sugiyama Y (2004) Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res 21(10):1895–1903
Liu Z, Liu K (2013) The transporters of intestinal tract and techniques applied to evaluate interactions between drugs and transporters. Asian J Pharm Sci 8:151–158
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Malfertheiner P, Chan FKL, McColl KEL (2009) Peptic ulcer disease. Lancet 374:1449–1461
Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, van De Vijver MJ, Scheper RJ, Schellens JH (2001) Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 61(8):3458–3464
Mao Q (2008) BCRP/ABCG2 in the placenta: expression, function and regulation. Pharm Res 25(6):1244–1255
Mao Q, Unadkat JD (2005) Role of the breast cancer resistance protein (ABCG2) in drug transport. AAPS J 7(1):E118–E133
Mizuarai S, Aozasa N, Kotani H (2004) Single nucleotide polymorphisms result in impaired membrane localization and reduced ATPase activity in multidrug transporter ABCG2. Int J Cancer 109:238–246
Mo W, Zhang JT (2012) Human ABCG2: structure, function, and its role in multidrug resistance. Int J Biochem Mol Biol 3(1):1–27
Morisaki K, Robey RW, Ozvegy-Laczka C, Honjo Y, Polgar O, Steadman K, Sarkadi B, Bates SE (2005) Single nucleotide polymorphisms modify the transporter activity of ABCG2. Cancer Chemother Pharmacol 56:161–172
Pang L, Word B, Xu J, Wang H, Hammons G, Huang SM, Lyn-Cook B (2014) ATP-binding cassette genes genotype and expression: a potential association with pancreatic cancer development and chemoresistance? Gastroenterol Res Pract 2014:414931. doi:10.1155/2014/414931
Petrovic V, Kojovic D, Cressman A, Piquette-Miller M (2015) Maternal bacterial infections impact expression of drug transporters in human placenta. Int Immunopharmacol 26(2):349–356. doi:10.1016/j.intimp.2015.04.020
Prasad B, Lai Y, Lin Y, Unadkat JD (2013) Interindividual variability in the hepatic expression of the human breast cancer resistance protein (BCRP/ABCG2): effect of age, sex, and genotype. J Pharm Sci 102(3):787–793. doi:10.1002/jps.23436
Ramakrishnan K, Salinas RC (2007) Peptic ulcer disease. Am Fam Physician 76(7):1005–1012
Schabowski J (2002) Selected socio-economic features and the prevalence of peptic ulcer among Polish rural population. Ann Agric Environ Med 9(1):79–84
Sparreboom A, Loos WJ, Burger H, Sissung TM, Verweij J, Figg WD, Nooter K, Gelderblom H (2005) Effect of ABCG2 genotype on the oral bioavailability of topotecan. Cancer Biol Ther 4:650–658
Szyca R, Źródlewski R, Leksowski K (2012) Stenosis pylori—a case report. Prz Gastroenterol 7(3):173–175
Thorsen K, Søreide JA, Kvaløy JT, Glomsaker T, Søreide K (2013) Epidemiology of perforated peptic ulcer: age- and gender-adjusted analysis of incidence and mortality. World J Gastroenterol 19(3):347–354
Tyburski J, Studzinska A, Daca P, Tretyn A (2008) PCR in real time. The methods of data analysis. Biotechnologia 1:86–96
Zhou D, Liu Y, Zhang X, Gu X, Wang H, Luo X, Zhang J, Zou H, Guan M (2014) Functional polymorphisms of the ABCG2 gene are associated with gout disease in the Chinese Han male population. Int J Mol Sci 15:9149–9159
Acknowledgments
This research was supported by Statutory Funds of the Department of Pharmaceutical Biochemistry and Molecular Diagnostics, Medical University of Lodz 503/3-015-02/503-01 and Funds of the Faculty of Pharmacy, Medical University of Lodz 502-03/3-015-02/502-34-048 and 502-03/3-015-02/502-34-049.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Committee of the Medical University of Lodz (RNN/285/13/KE; RNN/195/13/KE) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by: Michal Witt
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Salagacka-Kubiak, A., Żebrowska, M., Wosiak, A. et al. ABCG2 in peptic ulcer: gene expression and mutation analysis. J Appl Genetics 57, 335–342 (2016). https://doi.org/10.1007/s13353-015-0327-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13353-015-0327-0