Abstract
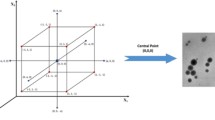
Disodium pamidronate, a second-generation bisphosphonate is a potent drug for the treatment of osteoporosis, which has been very well established by previous literature. It has very low oral permeability, leading to its low oral bioavailability, which restrict this drug to being administered orally. Therefore, the present research work includes the development of an orally effective nanoformulation of pamidronate. In this work, disodium pamidronate was complexed with phospholipon 90G for the enhancement of permeability and to investigate the phospholipon 90G–tagged pamidronate complex–loaded SNEDDS for oral delivery with promises of enhanced bioavailability and anti-osteoporotic activity. The rational design and optimization was employed using Central Composite Design (Design Expert® 12, software) to optimize nanoformulation parameters. In this work, a commercially potential self nano-emulsifying drug delivery system (SNEDDS) has been developed and evaluated for improved oral bioavailability and better clinical acceptance. The hot micro-emulsification and ultracentrifugation method with vortex mixing was utilized for effective tagging of phospholipon 90G with pamidronate and then loading into the SNEDDS nanocarrier. The optimized Pam-PLc SNEDDS formulation was characterized for particle size, PDI, and zeta potential and found to be 56.38 ± 1.37 nm, 0.218 ± 0.113, and 22.41 ± 1.14 respectively. Also, a 37.9% improved bioavailability of pamidronate compared to marketed tablet was observed. Similarly, in vivo pharmacokinetic studies suggest a 31.77% increased bone density and significant enhanced bone biomarkers compared to marketed tablets. The developed formulation is safe and effectively overcomes anti-osteoporosis promises with improved therapeutic potential. This work provides very significant achievements in postmenopausal osteoporosis treatment and may lead to possible use of nanotherapeutic-driven emerging biodegradable carriers-based drug delivery.
Graphical Abstract


















Similar content being viewed by others
Data availability
This paper contains all the data available as supplementary files.
References
Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–45.
Sun X, Wei J, Lyu J, Bian T, Liu Z, Huang J, et al. Bone-targeting drug delivery system of biomineral-binding liposomes loaded with icariin enhances the treatment for osteoporosis. J Nanobiotechnology BioMed Central. 2019;17:1–16. Available from: https://doi.org/10.1186/s12951-019-0447-5
Peasgood T, Herrmann K, Kanis JA, Brazier JE. An updated systematic review of health state utility values for osteoporosis related conditions. Osteoporos Int. 2009;20:853–68.
Eddy DM, Johnston J, Cummings SR, Dawson-Hughes B, Lindsay R, Melton LJ, et al. Osteoporosis: review of the evidence for prevention, diagnosis, and treatment and cost-effectiveness analysis. Status report Osteoporos Int. 1998;8:83–5.
Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Osteoporos Int. 1998;8 Suppl 4.
Hollick RJ, Reid DM. Role of bisphosphonates in the management of postmenopausal osteoporosis: an update on recent safety anxieties. Menopause Int. 2011;17:66–72.
Pazianas M, Miller P, Blumentals WA, Bernal M, Kothawala P. A Review of the literature on osteonecrosis of the jaw in patients with osteoporosis treated with oral bisphosphonates: prevalence, risk factors, and clinical characteristics. Clin Ther. 2007;29:1548–58.
Khajuria DK, Disha C, Vasireddi R, Razdan R, Mahapatra DR. Risedronate/zinc-hydroxyapatite based nanomedicine for osteoporosis. Mater Sci Eng C. Elsevier B.V. 2016;63:78–87. Available from: https://doi.org/10.1016/j.msec.2016.02.062
Maher M. Efficacy and safety of bisphosphonates for. 2014.
Widler L, Jaeggi KA, Glatt M, Müller K, Bachmann R, Bisping M, et al. Highly potent geminal bisphosphonates. From pamidronate disodium (Aredia) to zoledronic acid (Zometa). J Med Chem. 2002;45:3721–38.
Tissue C. Apposition and resorption of bone during oral treatment with (3-amino-1-hydroxypropylidene)-1,1-bisphosphonate (APD). 1983;357–61.
Njeh CF, Boivin CM, Langton CM. The role of ultrasound in the assessment of osteoporosis: a review. Osteoporos Int. 1997;7:7–22.
Fehlings D, Switzer L, Agarwal P, Wong C, Sochett E, Stevenson R, et al. Informing evidence-based clinical practice guidelines for children with cerebral palsy at risk of osteoporosis: a systematic review. Dev Med Child Neurol. 2012;54:106–16.
Panzavolta S, Torricelli P, Bracci B, Fini M, Bigi A. Alendronate and pamidronate calcium phosphate bone cements: setting properties and in vitro response of osteoblast and osteoclast cells. J Inorg Biochem. Elsevier Inc. 2009;103:101–6. Available from: https://doi.org/10.1016/j.jinorgbio.2008.09.012
Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis: recent developments and future perspectives. Biomark Res Biomarker Research. 2017;5:5–13.
Sparidans RW, Twiss IM, Talbot S. Bisphosphonates in bone diseases. Pharm World Sci. 1998;20:206–13.
Ralston SH, Alzaid AA, Gallacher SJ, Gardner MD, Cowan RA, Boyle IT. Clinical experience with aminohydroxypropylidene bisphosphonate (APD) in the management of cancer-associated hypercalcaemia. QJM. 1988;69:825–34.
Desai NS, Nagarsenker MS. Design and evaluation of self-nanoemulsifying pellets of repaglinide. AAPS PharmSciTech. 2013;14:994–1003.
Ruan J, Liu J, Zhu D, Gong T, Yang F, Hao X, et al. Preparation and evaluation of self-nanoemulsified drug delivery systems (SNEDDSs) of matrine based on drug-phospholipid complex technique. Int J Pharm. 2010;386:282–90.
Niamprem P, Rujivipat S, Tiyaboonchai W. Development and characterization of lutein-loaded SNEDDS for enhanced absorption in Caco-2 cells. Pharm Dev Technol. 2014;19:735–42.
Baloch J, Sohail MF, Sarwar HS, Kiani MH, Khan GM, Jahan S, et al. Self-nanoemulsifying drug delivery system (Snedds) for improved oral bioavailability of chlorpromazine: in vitro and in vivo evaluation. Med. 2019;55:1–13.
Mustapha O, Kim KS, Shafique S, Kim DS, Jin SG, Seo YG, et al. Development of novel cilostazol–loaded solid SNEDDS using a SPG membrane emulsification technique: physicochemical characterization and in vivo evaluation. Colloids Surfaces B Biointerfaces. Elsevier B.V. 2017;150:216–22. Available from: https://doi.org/10.1016/j.colsurfb.2016.11.039
Singh G, Pai RS. Optimized self-nanoemulsifying drug delivery system of atazanavir with enhanced oral bioavailability: in vitro/in vivo characterization. Expert Opin Drug Deliv. 2014;11:1023–32.
Solanki P, Singh R, Singh VD. Formulation and evaluation of fast dissolving tablet: a review. Solanki al World J Pharm Res. 2016;5.
Ma H, Chen H, Sun L, Tong L, Zhang T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia. Elsevier B.V. 2014;93:54–61. Available from: https://doi.org/10.1016/j.fitote.2013.10.016
Tiwari R, Kumar A, Solanki P, Dhobi M, Sundaresan V, Kalaiselvan V, et al. Analytical quality-by-design (AQbD) guided development of a robust HPLC method for the quantification of plumbagin from Plumbago species. J Liq Chromatogr Relat Technol. 2021.
Cui F, Shi K, Zhang L, Tao A, Kawashima Y. Biodegradable nanoparticles loaded with insulin-phospholipid complex for oral delivery: preparation, in vitro characterization and in vivo evaluation. J Control Release. 2006;114:242–50.
Hosny KM, Aljaeid BM. Sildenafil citrate as oral solid lipid nanoparticles: a novel formula with higher bioavailability and sustained action for treatment of erectile dysfunction. Expert Opin Drug Deliv. 2014;11:1015–22.
Čerpnjak K, Zvonar A, Gašperlin M, Vrečer F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013;63:427–45.
Shakeel F, Haq N, Ali M, Alanazi FK, Alsarra IA. Impact of viscosity and refractive index on droplet size and zeta potential of model O/W and W/O nanoemulsion. Curr Nanosci. 2013;9:248–53.
Wong JA, Renton KW, Crocker JFS, O’Regan PA, Acott PD. Determination of pamidronate in human whole blood and urine by reversed-phase HPLC with fluorescence detection. Biomed Chromatogr. 2004;18:98–101.
Cremers S, Sparidans R, Den Hartigh J, Hamdy N, Vermeij P, Papapoulos S. A pharmacokinetic and pharmacodynamic model for intravenous bisphosphonate (pamidronate) in osteoporosis. Eur J Clin Pharmacol. 2002;57:883–90.
Bhandare P, Rao BM, Madhavan P, Someswararao N. A validated stability-indicating method for the determination of related substances and assay of pamidronate sodium pentahydrate by hplc without derivatization. Rasayan J Chem. 2010;3:87–93.
Khajuria DK, Razdan R, Mahapatra DR. Description of a new method of ovariectomy in female rats. Rev Bras Reumatol. 2012;52:462–70.
Lelovas PP, Xanthos TT, Thorma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58:424–30.
Hayatullina Z, Muhammad N, Mohamed N, Soelaiman I. Virgin Coconut Oil Supplementation Prevents Bone Loss in Osteoporosis Rat Model. 2012;2012.
Bonjour JP, Ammann P, Rizzoli R. Importance of preclinical studies in the development of drugs for treatment of osteoporosis: a review related to the 1998 WHO guidelines. Osteoporos Int. 1999;9:379–93.
Vijayan V, Khandelwal M, Manglani K, Gupta S, Surolia A. Methionine down-regulates TLR4/MyD88/NF-κB signalling in osteoclast precursors to reduce bone loss during osteoporosis. Br J Pharmacol. 2014;171:107–21.
Zakir F, Ahmad A, Farooq U, Mirza MA, Tripathi A, Singh D, et al. Design and development of a commercially viable in situ nanoemulgel for the treatment of postmenopausal osteoporosis. Nanomedicine. 2020;15:1167–87.
Solanki P, Ansari MD, Anjali, Khan I, Jahan RN, Nikita, et al. Repurposing pentosan polysulfate sodium as hyaluronic acid linked polyion complex nanoparticles for the management of osteoarthritis: a potential approach. Med Hypotheses. 2021;157.
Mukherjee D, Srinivasan B, Anbu J, Azamthulla M, Teja BV, Ramachandra SG, et al. Pamidronate functionalized mucoadhesive compact for treatment of osteoporosis-in vitro and in vivo characterization. J Drug Deliv Sci Technol. Elsevier. 2019;52:915–26. Available from: https://doi.org/10.1016/j.jddst.2019.06.001
Shah FA, Ruscsák K, Palmquist A. 50 years of scanning electron microscopy of bone—a comprehensive overview of the important discoveries made and insights gained into bone material properties in health, disease, and taphonomy. Bone Res. Springer US. 2019;7:1–15. Available from: https://doi.org/10.1038/s41413-019-0053-z
Papapoulos SE. Bisphosphonates in the management of postmenopausal osteoporosis. Osteoporosis. 2001;631–50.
Kim KS, Yang ES, Kim DS, Kim DW, Yoo HH, Yong CS, et al. A novel solid self-nanoemulsifying drug delivery system (S-snedds) for improved stability and oral bioavailability of an oily drug, 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol. Drug Deliv. Informa Healthcare USA, Inc. 2017;24:1018–25. Available from: https://doi.org/10.1080/10717544.2017.1344335
Ansari MD, khan I, Solanki P, Pandit J, Jahan RN, Aqil M, et al. Fabrication and optimization of raloxifene loaded spanlastics vesicle for transdermal delivery. J Drug Deliv Sci Technol. Elsevier B.V. 2022;68:103102. Available from: https://doi.org/10.1016/j.jddst.2022.103102
Ansari MD, Saifi Z, Pandit J, Khan I, Solanki P, Sultana Y, et al. Spanlastics a novel nanovesicular carrier: its potential application and emerging trends in therapeutic delivery. AAPS PharmSciTech. AAPS PharmSciTech. 2022;23.
Upadhye SB, Kulkarni SJ, Majumdar S, Avery MA, Gul W, Elsohly MA, et al. Preparation and characterization of inclusion complexes of a hemisuccinate ester prodrug of Δ9-tetrahydrocannabinol with modified beta-cyclodextrins. AAPS PharmSciTech. 2010;11:509–17.
Renny JS, Tomasevich LL, Tallmadge EH, Collum DB. Method of continuous variations: applications of job plots to the study of molecular associations in organometallic chemistry. Angew Chemie - Int Ed. 2013;52:11998–2013.
Hirose K. A practical guide for the determination of binding constants. J Incl Phenom. 2001;39:193–209.
Saoji SD, Raut NA, Dhore PW, Borkar CD, Popielarczyk M, Dave VS. Preparation and evaluation of phospholipid-based complex of standardized Centella extract (SCE) for the enhanced delivery of phytoconstituents. AAPS J. 2016;18:102–14.
Ndamase AS, Aderibigbe BA, Sadiku ER, Labuschagne P, Lemmer Y, Ray SS, et al. Synthesis, characterization and in vitro cytotoxicity evaluation of polyamidoamine conjugate containing pamidronate and platinum drug. J Drug Deliv Sci Technol. Elsevier. 2018;43:267–73. Available from: https://doi.org/10.1016/j.jddst.2017.10.011
Dora CP, Kushwah V, Katiyar SS, Kumar P, Pillay V, Suresh S, et al. Improved oral bioavailability and therapeutic efficacy of erlotinib through molecular complexation with phospholipid. Int J Pharm. Elsevier. 2017;534:1–13. Available from: https://doi.org/10.1016/j.ijpharm.2017.09.071
Luo E, Hu J, Li JH, He G, Wei SC, Cui J. Bisphosphonate pamidronate modifying hydroxyapatite bioceramics for hard tissue repair. Key Eng Mater. 2007;336–338:1711–4.
Spickett CM, Pitt AR. Oxidative lipidomics coming of age: advances in analysis of oxidized phospholipids in physiology and pathology. Antioxidants Redox Signal. 2015;22:1646–66.
Khurana RK, Bansal AK, Beg S, Burrow AJ, Katare OP, Singh KK, et al. Enhancing biopharmaceutical attributes of phospholipid complex-loaded nanostructured lipidic carriers of mangiferin: systematic development, characterization and evaluation. Int J Pharm. 2017;518:289–306.
Hou Z, Li Y, Huang Y, Zhou C, Lin J, Wang Y, et al. Phytosomes loaded with mitomycin C-soybean phosphatidylcholine complex developed for drug delivery. Mol Pharm. 2013;10:90–101.
Fernandes C, Leite RS, Lanças FM. Rapid determination of bisphosphonates by ion chromatography with indirect UV detection. J Chromatogr Sci. 2007;45:236–41.
Shah RB, Zidan AS, Funck T, Tawakkul MA, Nguyenpho A, Khan MA. Quality by design: characterization of self-nano-emulsified drug delivery systems (SNEDDs) using ultrasonic resonator technology. Int J Pharm. 2007;341:189–94.
Bandyopadhyay S, Katare OP, Singh B. Optimized self nano-emulsifying systems of ezetimibe with enhanced bioavailability potential using long chain and medium chain triglycerides. Colloids Surfaces B Biointerfaces. Elsevier B.V. 2012;100:50–61. Available from: https://doi.org/10.1016/j.colsurfb.2012.05.019
Nasr A, Gardouh A, Ghorab M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics. 2016;8.
Fatouros DG, Karpf DM, Nielsen FS, Mullertz A. Clinical studies with oral lipid based formulations of poorly soluble compounds. Ther Clin Risk Manag. 2007;3:591–604.
Telange DR, Sohail NK, Hemke AT, Kharkar PS, Pethe AM. Phospholipid complex-loaded self-assembled phytosomal soft nanoparticles: evidence of enhanced solubility, dissolution rate, ex vivo permeability, oral bioavailability, and antioxidant potential of mangiferin. Drug Deliv Transl Res. 2021;11:1056–83.
Telange DR. Drug-phospholipid complex-loaded matrix film formulation for the enhanced transdermal delivery of quercetin how has open access to Fisher Digital Publications benefited you ? Drug-phospholipid complex-loaded matrix film formulation for the enhanced. 2018;31–50.
Moolakkadath T, Aqil M, Ahad A, Imam SS, Praveen A, Sultana Y, et al. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int J Pharm. 2019;560:78–91.
Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015;22:552–61.
Nauli AM, Whittimore JD. Using caco-2 cells to study lipid transport by the intestine. J Vis Exp. 2015;2015:1–8.
Khan I, Pandit J, Ahmed S, Zameer S, Nikita, Ahmad S, et al. Development and evaluation of biodegradable polymeric lomustine nanofibres for the efficient tumor targeting: in vitro characterization, ex vivo permeation and degradation study. J Drug Deliv Sci Technol. Elsevier B.V. 2022;75:103685. Available from: https://doi.org/10.1016/j.jddst.2022.103685
Solanki P, Sultana Y, Singh S. Traditional medicine: exploring their potential in overcoming multi-drug resistance. Strateg to Overcome Superbug Invasions Emerg Res Oppor. 2021;118–29.
Abo Enin HA, Abdel-Bar HM. Solid super saturated self-nanoemulsifying drug delivery system (sat-SNEDDS) as a promising alternative to conventional SNEDDS for improvement rosuvastatin calcium oral bioavailability. Expert Opin Drug Deliv. 2016;13:1513–21.
Ahmed S, Gull A, Alam M, Aqil M, Sultana Y. Ultrasonically tailored, chemically engineered and “QbD” enabled fabrication of agomelatine nanoemulsion; optimization, characterization, ex-vivo permeation and stability study. Ultrason Sonochem. Elsevier. 2018;41:213–26. Available from: https://doi.org/10.1016/j.ultsonch.2017.09.042
Mirza MA, Rahman MA, Talegaonkar S, Iqbal Z. In vitro/in vivo performance of different complexes of itraconazole used in the treatment of vaginal candidiasis. Brazilian J Pharm Sci. 2012;48:759–72.
Acknowledgements
Author highly acknowledges the financial support of Nano Mission, Department of Science and Technology, Govt. of India, bearing the file no. SR/NM/NS-1162/2015.
Funding
Author received complete financial assistance from Dept. of Science and Technology, New Delhi, file so. SR/NM/NS-1162/2015.
Author information
Authors and Affiliations
Contributions
Pavitra Solanki: writing, concept, methodology and final draft; Mohd. Danish Ansari: methodology, review; Mohd. Aqil: reviewing, concept; Farhan J. Ahmad: evaluation, methodology; Yasmin Sultana: supervision, evaluation and final draft.
Corresponding author
Ethics declarations
Ethical statement
I conducted animal studies myself with integrity, fidelity, and honesty. I openly take responsibility for my actions, and only make agreements, which I intend to keep. I did not intentionally engage in or participate in any form of malicious harm to another person or animal. Prior approval was taken from the institutional animal ethical committee for use of female wistar rats.
Ethics approval and consent to participate
This research work was approved by Institutional Animal Ethics Committee, Jamia Hamdard, New Delhi wide animal protocol no. 1726.
Consent for publication
Author declares that they have provided their consent for publishing this manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solanki, P., Ansari, M.D., Alam, M.I. et al. Precision engineering designed phospholipid-tagged pamidronate complex functionalized SNEDDS for the treatment of postmenopausal osteoporosis. Drug Deliv. and Transl. Res. 13, 883–913 (2023). https://doi.org/10.1007/s13346-022-01259-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01259-7