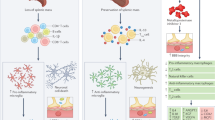
Abstract
Cell-based therapies to the brain are promising for the treatment of multiple brain disorders including neurodegeneration and cancers. In order to access the brain parenchyma, there are multiple physiological barriers that must be overcome depending on the route of delivery. Specifically, the blood–brain barrier has been a major difficulty in drug delivery for decades, and it still presents a challenge for the delivery of therapeutic cells. Other barriers, including the blood-cerebrospinal fluid barrier and lymphatic-brain barrier, are less explored, but may offer specific challenges or opportunities for therapeutic delivery. Here we discuss the barriers to the brain and the strategies currently in place to deliver cell-based therapies, including engineered T cells, dendritic cells, and stem cells, to treat diseases. With a particular focus on cancers, we also highlight the current ongoing clinical trials that use cell-based therapies to treat disease, many of which show promise at treating some of the deadliest illnesses.
Graphical abstract




Similar content being viewed by others
References
Weber EW, Maus MV, Mackall CL. The emerging landscape of immune cell therapies. Cell. 2020;181(1):46–62. https://doi.org/10.1016/j.cell.2020.03.001.
Hu X, Leak RK, Thomson AW, Yu F, Xia Y, Wechsler LR, Chen J. Promises and limitations of immune cell-based therapies in neurological disorders. Nat Rev Neurol. 2018;14(9):559–68. https://doi.org/10.1038/s41582-018-0028-5.
Akhavan D, Alizadeh D, Wang D, Weist MR, Shepphird JK, Brown CE. CAR T cells for brain tumors: lessons learned and road ahead. Immunol Rev. 2019;290(1):60–84. https://doi.org/10.1111/imr.12773.
Chen Y, Yu Z, Tan X, Jiang H, Xu Z, Fang Y, Han D, Hong W, Wei W, Tu J. CAR-Macrophage: a new immunotherapy candidate against solid tumors. Biomed Pharmacother. 2021;139: 111605. https://doi.org/10.1016/j.biopha.2021.111605.
Kim W, Liau LM. Dendritic cell vaccines for brain tumors. Neurosurg Clin N Am. 2010;21(1):139–57. https://doi.org/10.1016/j.nec.2009.09.005.
De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, Sciot R, Wilms G, Demaerel P, Warmuth-Metz M, Soerensen N, Wolff JEA, Wagner S, Kaempgen E, Van Gool SW. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res Off J Am Assoc Cancer Res. 2008;14(10):3098–3104. https://doi.org/10.1158/1078-0432.CCR-07-4875.
Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, Yoshida S, Abe T, Narita M, Takahashi M, Tanaka R. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11(11):4160–4167. https://doi.org/10.1158/1078-0432.CCR-05-0120.
Rutkowski S, De Vleeschouwer S, Kaempgen E, Wolff JEA, Kühl J, Demaerel P, Warmuth-Metz M, Flamen P, Van Calenbergh F, Plets C, Sörensen N, Opitz A, Van Gool SW. Surgery and adjuvant dendritic cell-based tumour vaccination for patients with relapsed malignant glioma, a feasibility study. Br J Cancer. 2004;91(9):1656–62. https://doi.org/10.1038/sj.bjc.6602195.
De Vleeschouwer S, Van Calenbergh F, Demaerel P, Flamen P, Rutkowski S, Kaempgen E, Wolff JE, Plets C, Sciot R, Van Gool SW. Transient local response and persistent tumor control in a child with recurrent malignant glioma: treatment with combination therapy including dendritic cell therapy. Case report J Neurosurg. 2004;100(5 Suppl Pediatrics):492–497. https://doi.org/10.3171/ped.2004.100.5.0492.
Barratt-Boyes SM, Zimmer MI, Harshyne LA, Meyer EM, Watkins SC, Capuano S, Murphey-Corb M, Falo LD, Donnenberg AD. Maturation and trafficking of monocyte-derived dendritic cells in monkeys: implications for dendritic cell-based vaccines. J Immunol Baltim Md 1950. 2000;164(5):2487–95.
Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008;15(10):739–52. https://doi.org/10.1038/gt.2008.41.
Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8(6):1481–93. https://doi.org/10.7150/thno.21254.
Pehlivan SB. Nanotechnology-based drug delivery systems for targeting, imaging and diagnosis of neurodegenerative diseases. Pharm Res. 2013;30(10):2499–511. https://doi.org/10.1007/s11095-013-1156-7.
Guerra M, Blázquez JL, Rodríguez EM. Blood-brain barrier and foetal-onset hydrocephalus, with a view on potential novel treatments beyond managing CSF flow. Fluids Barriers CNS. 2017;14(1):19. https://doi.org/10.1186/s12987-017-0067-0.
Komarova YA, Kruse K, Mehta D, Malik AB. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ Res. 2017;120(1):179–206. https://doi.org/10.1161/CIRCRESAHA.116.306534.
Lécuyer M-A, Saint-Laurent O, Bourbonnière L, Larouche S, Larochelle C, Michel L, Charabati M, Abadier M, Zandee S, Haghayegh Jahromi N, Gowing E, Pittet C, Lyck R, Engelhardt B, Prat A. Dual role of ALCAM in neuroinflammation and blood-brain barrier homeostasis. Proc Natl Acad Sci U S A. 2017;114(4):E524–33. https://doi.org/10.1073/pnas.1614336114.
Aday S, Cecchelli R, Hallier-Vanuxeem D, Dehouck MP, Ferreira L. Stem cell-based human blood–brain barrier models for drug discovery and delivery. Trends Biotechnol. 2016;34(5):382–93. https://doi.org/10.1016/j.tibtech.2016.01.001.
Warren KE. Beyond the blood:brain barrier: the importance of central nervous system (CNS) pharmacokinetics for the treatment of CNS tumors, Including Diffuse Intrinsic Pontine Glioma. Front Oncol. 2018;8. https://doi.org/10.3389/fonc.2018.00239.
Ghersi-Egea J-F, Strazielle N, Catala M, Silva-Vargas V, Doetsch F, Engelhardt B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol (Berl). 2018;135(3):337–61. https://doi.org/10.1007/s00401-018-1807-1.
Strominger I, Elyahu Y, Berner O, Reckhow J, Mittal K, Nemirovsky A, Monsonego A. The choroid plexus functions as a niche for T-cell stimulation within the central nervous system. Front Immunol. 2018;9. https://doi.org/10.3389/fimmu.2018.01066.
Wiatr M, Stump-Guthier C, Latorre D, Uhlig S, Weiss C, Ilonen J, Engelhardt B, Ishikawa H, Schwerk C, Schroten H, Tenenbaum T, Rudolph H. Distinct migratory pattern of naive and effector T cells through the blood–CSF barrier following echovirus 30 infection. J Neuroinflammation. 2019;16(1):232. https://doi.org/10.1186/s12974-019-1626-x.
Arvanitis CD, Askoxylakis V, Guo Y, Datta M, Kloepper J, Ferraro GB, Bernabeu MO, Fukumura D, McDannold N, Jain RK. Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood–tumor barrier disruption. Proc Natl Acad Sci. 2018;115(37):E8717–26. https://doi.org/10.1073/pnas.1807105115.
Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell. 2014;26(5):605–22. https://doi.org/10.1016/j.ccell.2014.10.006.
Kabraji S, Ni J, Lin NU, Xie S, Winer EP, Zhao JJ. Drug resistance in HER2- positive breast cancer brain metastases: blame the barrier or the brain? Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(8):1795–804. https://doi.org/10.1158/1078-0432.CCR-17-3351.
Kodack DP, Askoxylakis V, Ferraro GB, Sheng Q, Badeaux M, Goel S, Qi X, Shankaraiah R, Cao ZA, Ramjiawan RR, Bezwada D, Patel B, Song Y, Costa C, Naxerova K, Wong CSF, Kloepper J, Das R, Tam A, Tanboon J, Duda DG, Miller CR, Siegel MB, Anders CK, Sanders M, Estrada MV, Schlegel R, Arteaga CL, Brachtel E, Huang A, Fukumura D, Engelman JA, Jain RK. The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation. Sci Transl Med. 2017;9(391). https://doi.org/10.1126/scitranslmed.aal4682.
Askoxylakis V, Ferraro GB, Kodack DP, Badeaux M, Shankaraiah RC, Seano G, Kloepper J, Vardam T, Martin JD, Naxerova K, Bezwada D, Qi X, Selig MK, Brachtel E, Duda DG, Huang P, Fukumura D, Engelman JA, Jain RK. Preclinical efficacy of ado-trastuzumab emtansine in the brain microenvironment. J Natl Cancer Inst. 2016;108(2). https://doi.org/10.1093/jnci/djv313.
Lockman PR, Mittapalli RK, Taskar KS, Rudraraju V, Gril B, Bohn KA, Adkins CE, Roberts A, Thorsheim HR, Gaasch JA, Huang S, Palmieri D, Steeg PS, Smith QR. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(23):5664–78. https://doi.org/10.1158/1078-0432.CCR-10-1564.
Semyachkina-Glushkovskaya O, Postnov D, Kurths J. Blood–brain barrier, lymphatic clearance, and recovery: Ariadne’s thread in labyrinths of hypotheses. Int J Mol Sci. 2018;19(12). https://doi.org/10.3390/ijms19123818.
Caversaccio M, Peschel O, Arnold W. Connections between the cerebrospinal fluid space and the lymphatic system of the head and neck in humans. In: Ernst A, Marchbanks R, Samii M, editors. Intracranial and Intralabyrinthine Fluids. Berlin Heidelberg: Springer; 1996. p. 123–8. https://doi.org/10.1007/978-3-642-80163-1_15.
Si J, Chen L, Xia Z. Effects of cervical-lymphatic blockade on brain edema and infarction volume in cerebral ischemic rats. Chin J Physiol. 2006;49(5):258–65.
Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, Alitalo K, Thomas J-L, Iwasaki A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577(7792):689–94. https://doi.org/10.1038/s41586-019-1912-x.
Gutova M, Flores L, Adhikarla V, Tsaturyan L, Tirughana R, Aramburo S, Metz M, Gonzaga J, Annala A, Synold TW, Portnow J, Rockne RC, Aboody KS. Quantitative evaluation of intraventricular delivery of therapeutic neural stem cells to orthotopic glioma. Front Oncol. 2019;9. https://doi.org/10.3389/fonc.2019.00068.
Cohen-Pfeffer JL, Gururangan S, Lester T, Lim DA, Shaywitz AJ, Westphal M, Slavc I. Intracerebroventricular delivery as a safe, long-term route of drug administration. Pediatr Neurol. 2017;67:23–35. https://doi.org/10.1016/j.pediatrneurol.2016.10.022.
Donovan LK, Delaidelli A, Joseph SK, Bielamowicz K, Fousek K, Holgado BL, Manno A, Srikanthan D, Gad AZ, Van Ommeren R, Przelicki D, Richman C, Ramaswamy V, Daniels C, Pallota JG, Douglas T, Joynt ACM, Haapasalo J, Nor C, Vladoiu MC, Kuzan-Fischer CM, Garzia L, Mack SC, Varadharajan S, Baker ML, Hendrikse L, Ly M, Kharas K, Balin P, Wu X, Qin L, Huang N, Stucklin AG, Morrissy AS, Cavalli FMG, Luu B, Suarez R, De Antonellis P, Michealraj A, Rastan A, Hegde M, Komosa M, Sirbu O, Kumar SA, Abdullaev Z, Faria CC, Yip S, Hukin J, Tabori U, Hawkins C, Aldape K, Daugaard M, Maris JM, Sorensen PH, Ahmed N, Taylor MD. Locoregional delivery of CAR T cells to the cerebrospinal fluid for treatment of metastatic medulloblastoma and ependymoma. Nat Med. 2020;26(5):720–31. https://doi.org/10.1038/s41591-020-0827-2.
Theruvath J, Sotillo E, Mount CW, Graef CM, Delaidelli A, Heitzeneder S, Labanieh L, Dhingra S, Leruste A, Majzner RG, Xu P, Mueller S, Yecies DW, Finetti MA, Williamson D, Johann PD, Kool M, Pfister S, Hasselblatt M, Frühwald MC, Delattre O, Surdez D, Bourdeaut F, Puget S, Zaidi S, Mitra SS, Cheshier S, Sorensen PH, Monje M, Mackall CL. Locoregionally administered B7–H3-targeted CAR T cells for treatment of atypical teratoid/rhabdoid tumors. Nat Med. 2020;26(5):712–9. https://doi.org/10.1038/s41591-020-0821-8.
Brown CE, Aguilar B, Starr R, Yang X, Chang W-C, Weng L, Chang B, Sarkissian A, Brito A, Sanchez JF, Ostberg JR, D’Apuzzo M, Badie B, Barish ME, Forman SJ. Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther J Am Soc Gene Ther. 2018;26(1):31–44. https://doi.org/10.1016/j.ymthe.2017.10.002.
Weist MR, Starr R, Aguilar B, Chea J, Miles JK, Poku E, Gerdts E, Yang X, Priceman SJ, Forman SJ, Colcher D, Brown CE, Shively JE. PET of adoptively transferred chimeric antigen receptor T cells with 89Zr-Oxine. J Nucl Med Off Publ Soc Nucl Med. 2018;59(10):1531–7. https://doi.org/10.2967/jnumed.117.206714.
Goff SL, Morgan RA, Yang JC, Sherry RM, Robbins PF, Restifo NP, Feldman SA, Lu Y-C, Lu L, Zheng Z, Xi L, Epstein M, McIntyre LS, Malekzadeh P, Raffeld M, Fine HA, Rosenberg SA. Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. J Immunother Hagerstown Md 1997. 2019;42(4):126–35. https://doi.org/10.1097/CJI.0000000000000260.
Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther J Am Soc Gene Ther. 2010;18(4):843–51. https://doi.org/10.1038/mt.2010.24.
Priceman SJ, Tilakawardane D, Jeang B, Aguilar B, Murad JP, Park AK, Chang W-C, Ostberg JR, Neman J, Jandial R, Portnow J, Forman SJ, Brown CE. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2+ breast cancer metastasis to the brain. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(1):95–105. https://doi.org/10.1158/1078-0432.CCR-17-2041.
Hong JJ, Rosenberg SA, Dudley ME, Yang JC, White DE, Butman JA, Sherry RM. Successful Treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res Off J Am Assoc Cancer Res. 2010;16(19):4892–8. https://doi.org/10.1158/1078-0432.CCR-10-1507.
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet Lond Engl. 2015;385(9967):517–28. https://doi.org/10.1016/S0140-6736(14)61403-3.
Abramson JS, McGree B, Noyes S, Plummer S, Wong C, Chen Y-B, Palmer E, Albertson T, Ferry JA, Arrillaga-Romany IC. Anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma. N Engl J Med. 2017;377(8):783–4. https://doi.org/10.1056/NEJMc1704610.
Fong L, Brockstedt D, Benike C, Wu L, Engleman EG. Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol Baltim Md 1950. 2001;166(6):4254–9. https://doi.org/10.4049/jimmunol.166.6.4254.
Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol Baltim Md 1950. 2004;173(4):2353–61. https://doi.org/10.4049/jimmunol.173.4.2353.
De Vries IJM, Krooshoop DJEB, Scharenborg NM, Lesterhuis WJ, Diepstra JHS, Van Muijen GNP, Strijk SP, Ruers TJ, Boerman OC, Oyen WJG, Adema GJ, Punt CJA, Figdor CG. Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res. 2003;63(1):12–7.
Eggert AA, Schreurs MW, Boerman OC, Oyen WJ, de Boer AJ, Punt CJ, Figdor CG, Adema GJ. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res. 1999;59(14):3340–5.
Barratt-Boyes SM, Figdor CG. Current issues in delivering DCs for immunotherapy. Cytotherapy. 2004;6(2):105–10. https://doi.org/10.1080/14653240410005258.
Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–401.
Hingtgen S, Kasmieh R, Elbayly E, Nesterenko I, Figueiredo J-L, Dash R, Sarkar D, Hall D, Kozakov D, Vajda S, Fisher PB, Shah K. A first-generation multi-functional cytokine for simultaneous optical tracking and tumor therapy. PLoS ONE. 2012;7(7): e40234. https://doi.org/10.1371/journal.pone.0040234.
Hingtgen SD, Kasmieh R, van de Water J, Weissleder R, Shah K. A novel molecule integrating therapeutic and diagnostic activities reveals multiple aspects of stem cell-based therapy. Stem Cells Dayt Ohio. 2010;28(4):832–41. https://doi.org/10.1002/stem.313.
Hingtgen S, Figueiredo J-L, Farrar C, Duebgen M, Martinez-Quintanilla J, Bhere D, Shah K. Real-time multi-modality imaging of glioblastoma tumor resection and recurrence. J Neurooncol. 2013;111(2):153–61. https://doi.org/10.1007/s11060-012-1008-z.
Kauer TM, Figueiredo J-L, Hingtgen S, Shah K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat Neurosci. 2012;15(2):197–204. https://doi.org/10.1038/nn.3019.
Shah K, Hingtgen S, Kasmieh R, Figueiredo JL, Garcia-Garcia E, Martinez-Serrano A, Breakefield X, Weissleder R. Bimodal viral vectors and in vivo imaging reveal the fate of human neural stem cells in experimental glioma model. J Neurosci Off J Soc Neurosci. 2008;28(17):4406–13. https://doi.org/10.1523/JNEUROSCI.0296-08.2008.
Aboody KS, Najbauer J, Metz MZ, D’Apuzzo M, Gutova M, Annala AJ, Synold TW, Couture LA,Blanchard S, Moats RA, Garcia E, Aramburo S, Valenzuela VV, Frank RT, Barish ME, Brown CE, Kim SU, Badie B, Portnow J. Neural stem cell-mediated enzyme-prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013;5(184). https://doi.org/10.1126/scitranslmed.3005365.
Shah K, Bureau E, Kim D-E, Yang K, Tang Y, Weissleder R, Breakefield XO. Glioma therapy and real-time imaging of neural precursor cell migration and tumor regression. Ann Neurol. 2005;57(1):34–41. https://doi.org/10.1002/ana.20306.
Bagó JR, Sheets KT, Hingtgen SD. Neural stem cell therapy for cancer. Methods San Diego Calif. 2016;99:37–43. https://doi.org/10.1016/j.ymeth.2015.08.013.
Barish ME, Herrmann K, Tang Y, Argalian Herculian S, Metz M, Aramburo S, Tirughana R, Gutova M, Annala A, Moats RA, Goldstein L, Rockne RC, Gutierrez J, Brown CE, Ghoda L, Aboody KS. Human neural stem cell biodistribution and predicted tumor coverage by a diffusible therapeutic in a mouse glioma model. Stem Cells Transl Med. 2017;6(6):1522–32. https://doi.org/10.1002/sctm.16-0397.
Li G, Bonamici N, Dey M, Lesniak MS, Balyasnikova IV. Intranasal delivery of stem cell-based therapies for the treatment of brain malignancies. Expert Opin Drug Deliv. 2018;15(2):163–72. https://doi.org/10.1080/17425247.2018.1378642.
Lu M-H, Ji W-L, Chen H, Sun Y-Y, Zhao X-Y, Wang F, Shi Y, Hu Y-N, Liu B-X, Wu J-W, Xu D-E, Zheng J-W, Liu C-F, Ma Q-H. Intranasal transplantation of human neural stem cells ameliorates Alzheimer’s disease-like pathology in a mouse model. Front Aging Neurosci. 2021;13: 650103. https://doi.org/10.3389/fnagi.2021.650103.
Ii WHF. Noninvasive intranasal stem cells bypass the blood-brain barrier to target the brain to treat Parkinson’s disease, stroke, MS, brain tumors, cerebral ischemia, Alzheimer’s and other CNS disorders. J Nat Sci. 2015;1(1):23.
Potts MB, Silvestrini MT, Lim DA. Devices for cell transplantation into the central nervous system: design considerations and emerging technologies. Surg Neurol Int. 2013;4(Suppl 1):S22–30. https://doi.org/10.4103/2152-7806.109190.
Jahangiri A, Chin AT, Flanigan PM, Chen R, Bankiewicz K, Aghi MK. Convection-enhanced delivery in glioblastoma: a review of preclinical and clinical studies. J Neurosurg. 2017;126(1):191–200. https://doi.org/10.3171/2016.1.JNS151591.
Rosenbluth KH, Martin AJ, Bringas J, Bankiewicz KS. Evaluation of pressure-driven brain infusions in nonhuman primates by intra-operative 7 tesla MRI. J Magn Reson Imaging. 2012;36(6):1339–46. https://doi.org/10.1002/jmri.23771.
Mehta AM, Sonabend AM, Bruce JN. Convection-enhanced delivery. Neurotherapeutics. 2017;14(2):358–71. https://doi.org/10.1007/s13311-017-0520-4.
Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47(12):3039–51.
Atik AF, Suryadevara CM, Schweller RM, West JL, Healy P, Ii JEH, Congdon KL, Sanchez-Perez L, McLendon RE, Archer GE, Fecci P, Sampson JH. Hyaluronic acid based low viscosity hydrogel as a novel carrier for convection enhanced delivery of CAR T cells. J Clin Neurosci. 2018;56:163–8. https://doi.org/10.1016/j.jocn.2018.06.005.
Zhang T, Murphy MJ, Yu H, Bagaria HG, Yoon KY, Neilson BM, Bielawski CW, Johnston KP, Huh C, Bryant SL. Investigation of nanoparticle adsorption during transport in porous media. SPE J. 2015;20(04):667–77. https://doi.org/10.2118/166346-PA.
Morrison PF, Chen MY, Chadwick RS, Lonser RR, Oldfield EH. Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol. 1999;277(4):R1218-1229. https://doi.org/10.1152/ajpregu.1999.277.4.R1218.
Elenes EY, Mehta JN, Hsu F-C, Whitlow CT, Debinski W, Rossmeisl J, Tatter S, Rylander CG. Convection-enhanced arborizing catheter system improves local/regional delivery of infusates versus a single-port catheter in ex vivo porcine brain tissue. J Eng Sci Med Diagn Ther. 2020;4(011003). https://doi.org/10.1115/1.4048935.
Silvestrini MT, Yin D, Coppes VG, Mann P, Martin AJ, Larson PS, Starr PA, Gupta N, Panter SS, Desai TA, Lim DA. Radially branched deployment for more efficient cell transplantation at the scale of the human brain. Stereotact Funct Neurosurg. 2013;91(2):92–103. https://doi.org/10.1159/000343213.
Raghavan R, Odland RM. Theory of porous catheters and their applications in intraparenchymal infusions. Biomed Phys Eng Exp. 2017;3(2).
Brady M, Raghavan R, Sampson J. Determinants of intraparenchymal infusion distributions: modeling and analyses of human glioblastoma trials. Pharmaceutics. 2020;12(9):895. https://doi.org/10.3390/pharmaceutics12090895.
Raghavan R, Brady M. Predictive models for pressure-driven fluid infusions into brain parenchyma. Phys Med Biol. 2011;56(19):6179–204. https://doi.org/10.1088/0031-9155/56/19/003.
Woodall RT, Ii DAH, Abdelmalik MRA, Wu C, Feng X, Phillips WT, Bao A, Hughes TJR, Brenner AJ, Yankeelov TE. Integrating quantitative imaging and computational modeling to predict the spatiotemporal distribution of 186Re nanoliposomes for recurrent glioblastoma treatment. In Medical Imaging 2019 Physics of Medical Imaging. Int Soc Optics Photonics. 2019;10948:109483M. https://doi.org/10.1117/12.2512867.
Zhan W, Wang C-H. Convection enhanced delivery of chemotherapeutic drugs into brain tumour. J Controlled Release. 2018;271:74–87. https://doi.org/10.1016/j.jconrel.2017.12.020.
Vendel E, Rottschäfer V, de Lange ECM. The need for mathematical modelling of spatial drug distribution within the brain. Fluids Barriers CNS. 2019;16(1):12. https://doi.org/10.1186/s12987-019-0133-x.
Yu J, Berlin JM, Lu W, Zhang L, Kan AT, Zhang P, Walsh EE, Work SN, Chen W, Tour JM, Wong MS, Tomson MB. Transport study of nanoparticles for oilfield application. OnePetro. 2010. https://doi.org/10.2118/131158-MS.
Sahoo P, Yang X, Abler D, Maestrini D, Adhikarla V, Frankhouser D, Cho H, Machuca V, Wang D, Barish M, Gutova M, Branciamore S, Brown CE, Rockne RC. Mathematical deconvolution of CAR T-cell proliferation and exhaustion from real-time killing assay data. J R Soc Interface. 2020;17(162):20190734. https://doi.org/10.1098/rsif.2019.0734.
Kingsmore KM, Vaccari A, Abler D, Cui SX, Epstein FH, Rockne RC, Acton ST, Munson JM. MRI analysis to map interstitial flow in the brain tumor microenvironment. APL Bioeng. 2018;2(3): 031905. https://doi.org/10.1063/1.5023503.
Stine CA, Munson JM. Convection-enhanced delivery: connection to and impact of interstitial fluid flow. Front Oncol. 2019;9. https://doi.org/10.3389/fonc.2019.00966.
Zhan W, Baena FR. y; Dini, D. Effect of tissue permeability and drug diffusion anisotropy on convection-enhanced delivery. Drug Deliv. 2019;26(1);773–781. https://doi.org/10.1080/10717544.2019.1639844.
Chatterjee K, Atay N, Abler D, Bhargava S, Sahoo P, Rockne RC, Munson JM. Utilizing dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) to analyze interstitial fluid flow and transport in glioblastoma and the surrounding parenchyma in human patients. Pharmaceutics 2021;13(2). https://doi.org/10.3390/pharmaceutics13020212.
Jarrett AM, Faghihi D, Ii DAH, Lima EABF, Virostko J, Biros G, Patt D, Yankeelov TE. Optimal control theory for personalized therapeutic regimens in oncology: background, history, challenges, and opportunities. J Clin Med. 2020;9(5). https://doi.org/10.3390/jcm9051314.
Lenhart S, Workman JT. Optimal control applied to biological models. CRC Press, 2007.
Modelling optimization and control of biomedical systems. Pistikopoulos, E. N., Nascu, I., Velliou, E. G., Eds.; 2017.
Burgess A, Hynynen K. Drug delivery across the blood-brain barrier using focused ultrasound. Expert Opin Drug Deliv. 2014;11(5):711–21. https://doi.org/10.1517/17425247.2014.897693.
Alkins R, Burgess A, Ganguly M, Francia G, Kerbel R, Wels WS, Hynynen K. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73(6):1892–9. https://doi.org/10.1158/0008-5472.CAN-12-2609.
Alkins R, Burgess A, Kerbel R, Wels WS, Hynynen K. Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro-Oncol. 2016;18(7):974–81. https://doi.org/10.1093/neuonc/nov318.
Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS ONE. 2011;6(11): e27877. https://doi.org/10.1371/journal.pone.0027877.
Shen W-B, Anastasiadis P, Nguyen B, Yarnell D, Yarowsky PJ, Frenkel V, Fishman PS. Magnetic enhancement of stem cell-targeted delivery into the brain following MR-guided focused ultrasound for opening the blood-brain barrier. Cell Transplant. 2017;26(7):1235–46. https://doi.org/10.1177/0963689717715824.
Burgess A, Hynynen K. Drug Delivery across the Blood-Brain Barrier Using Focused Ultrasound. Expert Opin Drug Deliv. 2014;11(5):711–21. https://doi.org/10.1517/17425247.2014.897693.
Deng Z, Sheng Z, Yan F. Ultrasound-induced blood-brain-barrier opening enhances anticancer efficacy in the treatment of glioblastoma: current status and future prospects. J Oncol. 2019;2019: e2345203. https://doi.org/10.1155/2019/2345203.
Punganuru SR, Arutla V, Zhao W, Rajaei M, Deokar H, Zhang R, Buolamwini JK, Srivenugopal KS, Wang W. Targeted brain tumor therapy by inhibiting the MDM2 oncogene: in vitro and in vivo antitumor activity and mechanism of action. Cells. 2020;9(7):1592. https://doi.org/10.3390/cells9071592.
Wang D, Starr R, Chang W-C, Aguilar B, Alizadeh D, Wright SL, Yang X, Brito A, Sarkissian A, Ostberg JR, Li L, Shi Y, Gutova M, Aboody K, Badie B, Forman SJ, Barish ME, Brown CE. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci Transl Med 2020;12(533). https://doi.org/10.1126/scitranslmed.aaw2672.
Nonaka M, Suzuki-Anekoji M, Nakayama J, Mabashi-Asazuma H, Jarvis DL, Yeh J-C, Yamasaki K, Akama TO, Huang C-T, Campos AR, Nagaoka M, Sasai T, Kimura-Takagi I, Suwa Y, Yaegashi T, Shibata TK, Sugihara K, Nishizawa-Harada C, Fukuda M, Fukuda MN. Overcoming the blood–brain barrier by annexin A1-binding peptide to target brain tumours. Br J Cancer. 2020;123(11):1633–43. https://doi.org/10.1038/s41416-020-01066-2.
Suryadevara CM, Desai R, Abel ML, Riccione KA, Batich KA, Shen SH, Chongsathidkiet P, Gedeon PC, Elsamadicy AA, Snyder DJ, Herndon JE, Healy P, Archer GE, Choi BD, Fecci PE, Sampson JH, Sanchez-Perez L. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology. 2018;7(6): e1434464. https://doi.org/10.1080/2162402X.2018.1434464.
Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine 2020;59. https://doi.org/10.1016/j.ebiom.2020.102975.
PersonGen BioTherapeutics (Suzhou) Co., Ltd. Clinical investigation of chimeric CD(cluster of differentiation)33 antigen receptor-modified NK92 cells in relapsed and/or refractory acute myeloid leukemias. Clinical trial registration NCT02944162; clinicaltrials.gov, 2016.
Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, Yin J, You F, Zhu M, Shen W, Chen G, Zhu X, Wu D, Yu J. First-in-Man Clinical Trial of CAR NK-92 Cells: Safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8(6):1083–9.
The Third Affiliated Hospital of Guangzhou Medical University. Pilot study of NKG2D-ligand targeted CAR-NK cells in patients with metastatic solid tumours. Clinical trial registration NCT03415100; clinicaltrials.gov, 2018.
Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, Jin Q, Su L, Liu X, Wang K, Yan G, Dong T, Wu S, Zhou P, Zhang J, Liang W, Ren J, Teng Y, Chen C, Xu XH. Adoptive transfer of NKG2D CAR MRNA-engineered natural killer cells in colorectal cancer patients. Mol Ther J Am Soc Gene Ther. 2019;27(6):1114–25. https://doi.org/10.1016/j.ymthe.2019.03.011.
M.D. Anderson Cancer Center. Dose escalation study phase I/II of umbilical cord blood-derived CAR-engineered NK cells in conjunction with lymphodepleting chemotherapy in patients with relapsed/refractory B-lymphoid malignancies. Clinical trial registration NCT03056339; clinicaltrials.gov, 2020.
Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, Nassif Kerbauy L, Overman B, Thall P, Kaplan M, Nandivada V, Kaur I, Nunez Cortes A, Cao K, Daher M, Hosing C, Cohen EN, Kebriaei P, Mehta R, Neelapu S, Nieto Y, Wang M, Wierda W, Keating M, Champlin R, Shpall EJ, Rezvani K. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–53. https://doi.org/10.1056/NEJMoa1910607.
City of Hope Medical Center. A pilot feasibility study of oral 5-fluorocytosine and genetically-modified neural stem cells expressing E.coli cytosine deaminase for treatment of recurrent high grade gliomas. Clinical trial registration study/NCT01172964; clinicaltrials.gov, 2017.
Lesniak M. Neural stem cell oncolytic adenoviral virotherapy of newly diagnosed malignant glioma. Clinical trial registration NCT03072134; clinicaltrials.gov, 2020.
ReNeuron Limited. A randomized, placebo-controlled study of the efficacy and safety of intracerebral stem cells (CTX0E03) in subjects with disability following an ischemic stroke. Clinical trial registration NCT03629275; clinicaltrials.gov, 2020.
Safe Save Medical Cell Sciences & Technology Co., Ltd. Autologous dendritic cell/tumor antigen (ADCTA-SSI-G1) for adjuvant immunotherapy in standard treatment of recurrent glioblastoma multiforme (GBM): a multi-center, open-label, randomized phase III clinical trial. Clinical trial registration NCT04277221; clinicaltrials.gov, 2020.
Vik-Mo E. Open label randomized phase II/III trial of dendritic cell immunotherapy against cancer stem cells in glioblastoma patients receiving standard therapy (DEN-STEM). Clinical trial registration NCT03548571; clinicaltrials.gov, 2019.
Plosker GL. Sipuleucel-T: In metastatic castration-resistant prostate cancer. Drugs. 2011;71(1):101–8. https://doi.org/10.2165/11206840-000000000-00000.
Laus R, Ruegg CL, Wu H. Immunostimulatory composition. US6210662B1, April 3, 2001.
Richwine, L. UPDATE 3-U.S. FDA OKs Dendreon’s prostate cancer vaccine. Reuters. April 29, 2010.
Research, C. for B. E. and. PROVENGE (Sipuleucel-T). FDA 2019.
Research, C. for B. E. and. Approved products — PROVENGE (sipuleucel-T) https://wayback.archive-it.org/7993/20170722071307/https://www.fda.gov/BiologicsBloodVaccines/CellularGeneTherapyProducts/ApprovedProducts/ucm210012.htm (accessed 2021 -06 -08).
Drugs and Biologics Compendium https://www.nccn.org/compendia-templates/compendia/drugs-and-biologics-compendia (accessed 2021 -06 -08).
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. IMPACT study investigators. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5);411–422. https://doi.org/10.1056/NEJMoa1001294.
Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with Sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(19):3089–94. https://doi.org/10.1200/JCO.2005.04.5252.
PS1 late breaking immunotherapy (APC8015) for androgen independent prostate cancer (AIPC): final progression and survival data from a second phase 3 trial. Eur J Cancer Suppl. 2005;3(4):1. https://doi.org/10.1016/S1359-6349(05)82004-X.
New treatment options for patients with prostate cancer http://www.eurekalert.org/pub_releases/2005-11/foec-nto110205.php (accessed 2021 -06 -08).
Dendreon. Autologous PAP-loaded dendritic cell vaccine (Sipuleucel-T, APC8015, Provenge®) in patients with non-metastatic prostate cancer who experience PSA E levation following radical prostatectomy: a randomized, controlled, double-blind trial; Clinical trial registration NCT00779402; clinicaltrials.gov, 2017.
Prostate Oncology Specialists, Inc. Phase 1 study of Sipuleucel-T and Ipilimumab in combination for advanced prostate cancer; Clinical trial registration NCT01832870; clinicaltrials.gov, 2017.
Acknowledgements
Figures were created using Biorender with a license to JMM.
Funding
Funding provided to JMM from the National Cancer Institute R37CA222563. Funding provided to JMM, RCR, and CEB from the National Institute of Neurological Disorders and Stroke R01NS115971. Funding for OMT from the National Institute of General Medical Sciences R25GM066534 to Ed Smith.
Author information
Authors and Affiliations
Contributions
All authors wrote, edited, and developed the manuscript.
Corresponding author
Ethics declarations
Data availability
Not applicable.
Ethical statement
No animal or human studies were carried out by the authors for this article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Turk, O.M., Woodall, R.C., Gutova, M. et al. Delivery strategies for cell-based therapies in the brain: overcoming multiple barriers. Drug Deliv. and Transl. Res. 11, 2448–2467 (2021). https://doi.org/10.1007/s13346-021-01079-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01079-1