Abstract
Immunotherapies have been heavily explored in the last decade, ranging from new treatments for cancer to allergic diseases. These therapies target the immune system, a complex organ system consisting of tissues with intricate structures and cells with a multitude of functions. To better understand immune functions and develop better therapeutics, many cellular and 2-dimensional (2D) tissue models have been developed. However, research has demonstrated that the 3-dimensional (3D) tissue structure can significantly affect cellular functions, and this is not recapitulated by more traditional 2D models. Microfluidics has been used to design 3D tissue models that allow for intricate arrangements of cells and extracellular spaces, thus allowing for more physiologically relevant in vitro model systems. Here, we summarize the multitude of microfluidic devices designed to study the immune system with the ultimate goal to improve existing and design new immunotherapies. We have included models of the different immune organs, including bone marrow and lymph node (LN), models of immunity in diseases such as cancer and inflammatory bowel disease, and therapeutic models to test or engineer new immune-modulatory treatments. We particularly emphasize research on how microfluidic devices are used to better understand different physiological states and how interactions within the immune microenvironment can influence the efficacy of immunotherapies.
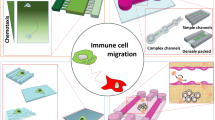
Graphical abstract








Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Consent for publication.
All the authors whose names appear on the submission (1) made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; (2) drafted the work or revised it critically for important intellectual content; (3) approved the version to be published; and (4) agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
References
The innate and adaptive immune systems. [Website] 2016 August 4, 2016; Available from: https://www.ncbi.nlm.nih.gov/books/NBK279396/.
Alberts BJA, Lewis J, et al. Innate immunity, in Molecular biology of the cell. Garland Science: New York. 2002.
Alberts BJA, Lewis J, et al. The adaptive immune system, in Molecular Biology of the Cell. Garland Science: New York. 2002.
Gwaltney-Brant S. Chapter 22 - Immunotoxicity biomarkers. In: Gupta RC, editor. Biomarkers in Toxicology. Boston: Academic Press; 2014. p. 373–85.
Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol (Baltimore, Md. : 1950), 2009;183(4):2205–2212.
Owen, J.A., et al. Kuby Immunology. New York: W.H. Freeman. 2013.
Janeway CA Jr, Walport TPM. et al. Generation of lymphocytes in bone marrow and thymus. Garland Sciences: New York. 2001.
Dunsmore SE, Shapiro SD. The bone marrow leaves its scar: new concepts in pulmonary fibrosis. J Clin Investig. 2004;113(2):180–2.
Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4(4):278–89.
Bujoreanu I, Gupta V. Anatomy, lymph nodes. 2020; Available from: https://www.ncbi.nlm.nih.gov/books/NBK557717/.
Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. Int Immunol. 2008;20(12):1483–7.
Willard-Mack CL. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006;34(5):409–24.
Drayton DL, et al. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–53.
Martinez VG, et al. Fibroblastic reticular cells control conduit matrix deposition during lymph node expansion. Cell Rep. 2019;29(9):2810-2822.e5.
Lewis SM, Williams A, Eisenbarth SC. Structure and function of the immune system in the spleen. Sci Immunol, 201;4(33).
Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol. 2006;34(5):599–608.
Bizzell E. Microbial ninja warriors: bacterial immune evasion. 2018.
Breslin JW. Mechanical forces and lymphatic transport. Microvasc Res. 2014;96:46–54.
Immunity in the tissues. Nat Immunol. 2013;14(10):977–977.
Fang P, et al. Immune cell subset differentiation and tissue inflammation. J Hematol Oncol. 2018;11(1):97–97.
Martinez-Chapa SO, Salazar A, Madou MJ. Chapter 13.2 - Two-photon polymerization as a component of desktop integrated manufacturing platforms, in Three-dimensional microfabrication using two-photon polymerization, T. Baldacchini, Editor. 2016, William Andrew Publishing: Oxford. 2016:374–416.
Rosato DV, Rosato MG. The complete injection molding process, in Injection Molding Handbook. Springer US, 2000;1460.
Weigl BH, et al. Design and rapid prototyping of thin-film laminate-based microfluidic devices. Biomed Microdevice. 2001;3(4):267–74.
Weibel DB, DiLuzio WR, Whitesides GM. Microfabrication meets microbiology. Nat Rev Microbiol. 2007;5(3):209–18.
Pocock K, et al. Intestine-on-a-chip microfluidic model for efficient in vitro screening of oral chemotherapeutic uptake. ACS Biomater Sci Eng. 2017;3(6):951–9.
Villenave R, et al. Human gut-on-a-chip supports polarized infection of Coxsackie B1 virus in vitro. PLoS One. 2017;12(2):e0169412.
Gjorevski N, et al. Neutrophilic infiltration in organ-on-a-chip model of tissue inflammation. Lab Chip. 2020;20(18):3365–74.
Beaurivage C, et al. Development of a gut-on-a-chip model for high throughput disease modeling and drug discovery. Int J Mol Sci. 2019;20(22):5661.
Bhatti FUR, Hasty KA, Cho H. Anti-inflammatory role of TPCA-1 encapsulated nanosomes in porcine chondrocytes against TNF-α stimulation. Inflammopharmacology. 2019;27(5):1011–9.
Ramadan Q, Gourikutty SBN, Zhang QX. OOCHIP: Compartmentalized microfluidic perfusion system with porous barriers for enhanced cell-cell crosstalk in organ-on-a-chip. Micromachines (Basel). 2020;11(6).
Huh D, et al. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986):1662.
Zamprogno P, et al. Second-generation lung-on-a-chip array with a stretchable biological membrane. bioRxiv, 2019:608919.
Benam KH, et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13(2):151–7.
Ross AE, Pompano RR. Diffusion of cytokines in live lymph node tissue using microfluidic integrated optical imaging. Anal Chim Acta. 2018;1000:205–13.
Ross AE, et al. Spatially resolved microfluidic stimulation of lymphoid tissue ex vivo. Analyst. 2017;142(4):649–59.
Shim S, et al. Two-way communication between ex vivo tissues on a microfluidic chip: application to tumor-lymph node interaction. Lab Chip. 2019;19(6):1013–26.
Haessler U, et al. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc Natl Acad Sci U S A. 2011;108(14):5614–9.
Kim S, et al. Multiscale engineering of immune cells and lymphoid organs. Nat Rev Mater. 2019;4(6):355–78.
Wagar LE, et al. Modeling human adaptive immune responses with tonsil organoids. Nat Med. 2021;27(1):125–35.
Aleman J. et al, Deconstructed microfluidic bone marrow on-a-chip to study normal and malignant hemopoietic cell-niche interactions. Small. 2019;15(43):e1902971.
Torisawa YS, et al. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods. 2014;11(6):663–9.
Chou DB, et al. On-chip recapitulation of clinical bone marrow toxicities and patient-specific pathophysiology. Nat Biomed Eng. 2020;4(4):394–406.
Lévesque JP, Helwani FM, Winkler IG. The endosteal ‘osteoblastic’ niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24(12):1979–92.
Tamma R, Ribatti D. Bone niches, hematopoietic stem cells, and vessel formation. Int J Mol Sci. 2017;18(1):151.
Oh M, Nör JE. The perivascular niche and self-renewal of stem cells. Front Physiol. 2015;6:367.
Charrot S, Hallam S. CAR-T cells: future perspectives. HemaSphere. 2019;3(2):e188.
Grosser R, et al. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36(5):471–82.
Tang N, et al. TGF-β inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI insight. 2020;5(4):e133977.
Wang, Y, et al. An IL-4/21 inverted cytokine receptor improving CAR-T cell potency in immunosuppressive solid-tumor microenvironment. Front Immunol. 2019;10(1691).
Zhang Z, et al. Modified CAR T cells targeting membrane-proximal epitope of mesothelin enhances the antitumor function against large solid tumor. Cell Death Dis. 2019;10(7):476.
Lugo-Cintrón KM, et al. Matrix density drives 3D organotypic lymphatic vessel activation in a microfluidic model of the breast tumor microenvironment. Lab Chip. 2020;20(9):1586–600.
Ren X, et al. Chapter nineteen - applications of microfluidic devices in advancing NK-cell migration studies, in Methods in Enzymology, L. Galluzzi and N.-P. Rudqvist, Editors. Academic Press. 2020;357–370.
Gerner MY, et al. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity. 2012;37(2):364–76.
Migliozzi D, et al. Microfluidics-assisted multiplexed biomarker detection for in situ mapping of immune cells in tumor sections. Microsyst Nanoeng. 2019;5(1):59.
Moore N, et al. A multiplexed microfluidic system for evaluation of dynamics of immune-tumor interactions. Lab Chip. 2018;18(13):1844–58.
Beckwith AL, Velásquez-García LF, Borenstein JT. Microfluidic model for evaluation of immune checkpoint inhibitors in human tumors. Adv Healthc Mater. 2019;8(11):e1900289.
Nunes AS, et al. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol Bioeng. 2019;116(1):206–26.
Jenkins RW, et al. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov. 2018;8(2):196–215.
Gunti S, et al. Organoid and spheroid tumor models: techniques and applications. Cancers. 2021;13(4):874.
Pinto B, et al. Three-dimensional spheroids as in vitro preclinical models for cancer research. Pharmaceutics. 2020;12(12).
Park D, et al. High-throughput microfluidic 3D cytotoxicity assay for cancer immunotherapy (CACI-IMPACT platform). Front Immunol. 2019;10(1133).
Park D, et al. High-throughput microfluidic 3D cytotoxicity assay for cancer immunotherapy (CACI-IMPACT platform). Front Immunol. 2019;10:1133.
Pavesi A, et al. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. JCI insight. 2017;2–12.
Perez CR, De Palma M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat Commun. 2019;10(1):5408.
Santini SM, et al. IFN-alpha in the generation of dendritic cells for cancer immunotherapy. Handb Exp Pharmacol. 2009;188:295–317.
Parlato S, et al. 3D Microfluidic model for evaluating immunotherapy efficacy by tracking dendritic cell behaviour toward tumor cells. Sci Rep. 2017;7(1):1093.
Moura Rosa P, et al. The intercell dynamics of T cells and dendritic cells in a lymph node-on-a-chip flow device. Lab Chip. 2016;16(19):3728–40.
Bounab Y, et al. Dynamic single-cell phenotyping of immune cells using the microfluidic platform DropMap. Nat Protoc. 2020;15(9):2920–55.
Tavakoli H, et al. Recent advances in microfluidic platforms for single-cell analysis in cancer biology, diagnosis and therapy. Trends in analytical chemistry. TrAC. 2019;117:13–26.
Segaliny AI, et al. Functional TCR T cell screening using single-cell droplet microfluidics. Lab Chip. 2018;18(24):3733–49.
Briones JC, et al. A microfluidic platform for single cell fluorometric Granzyme B profiling. Theranostics. 2020;10(1):123–32.
Funding
This study was funded by the American Lung Association Dalsemer Award (KM), LAM Foundation Career Development Award (KM), NIH T32 (5T32GM080201, MA), and MPower Maryland (KM).
Author information
Authors and Affiliations
Contributions
Conceptualization: Katharina Maisel. Literature search: Ann Ramirez, Mayowa Amosu, Priscilla Lee, and Katharina Maisel. Drafted the work: Ann Ramirez, Mayowa Amosu, and Priscilla Lee. Revised the work: Katharina Maisel and Ann Ramirez.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramirez, A., Amosu, M., Lee, P. et al. Microfluidic systems to study tissue barriers to immunotherapy. Drug Deliv. and Transl. Res. 11, 2414–2429 (2021). https://doi.org/10.1007/s13346-021-01016-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01016-2