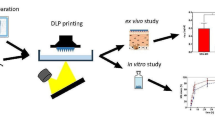
Abstract
Biodegradable polymeric microneedle arrays (BPMNAs) could be explored as potential devices for transdermal drug delivery, which can provide a painless and safe drug delivery method. BPMNAs could also provide high drug-loading capacity and prolonged drug delivery once integrated with a drug reservoir. However, the fabrication of MNAs with a drug reservoir is expensive and requires complicated procedures. The present study was conducted to describe the preparation of a reservoir-based BPMNA containing estradiol valerate using polylactic acid (PLA) with the combination of FDM 3D printing and injection volume filling techniques. The tip size of the 3D printed needles decreased to 173 μm utilizing a chemical etching process. The content of estradiol valerate loaded in the 3D printed PLA MNAs was 29.79 ± 0.03 mg, and the release was in a prolonged manner for up to 7 days. The results of mechanical tests revealed that the force needed for the 3D printed PLA MNAs fracture (900 N) was significantly higher than that needed for their skin penetration (4 N). The successful penetration of 3D printed PLA MNAs through the stratum corneum was confirmed via penetration test, methylene blue staining, and histological examination. The results showed that 3D printed PLA MNAs can penetrate into the skin without reaching to the dermal nerves and puncture of blood vessels. In conclusion, in the current study, we explored the practicability of the preparation of drug loaded reservoir-based BPMNAs using the combination of FDM 3D printing and injection volume filling techniques for painless and prolonged transdermal drug delivery.
Graphical abstract








Similar content being viewed by others
Code availability
Consent.
References
Gomez AM, Fuentes L, Allina A. Women or LARC First? Reproductive autonomy and the promotion of long-acting reversible contraceptive methods. Perspect Sex Reprod Health. 2014;46(3):171–5. https://doi.org/10.1363/46e1614.
Mishell DR, El-Habashy MA, Good RG, Moyer DL. Contraception with an injectable progestin. A study of its use in postpartum women. American Journal of Obstetrics and Gynecology. 1968;101(8):1046–53. https://doi.org/10.1016/0002-9378(68)90346-3.
Odom EB, Eisenberg DL, Fox IK. Difficult removal of subdermal contraceptive implants: a multidisciplinary approach involving a peripheral nerve expert. Contraception. 2017;96(2):89–95. https://doi.org/10.1016/j.contraception.2017.05.001.
Griffin JB, Ridgeway K, Montgomery E, Torjesen K, Clark R, Peterson J et al. Vaginal ring acceptability and related preferences among women in low- and middle-income countries: a systematic review and narrative synthesis. PLoS ONE. 2019;14(11). https://doi.org/10.1371/journal.pone.0224898.
Riemma Pierre M, Rossetti F. Microneedle-based drug delivery systems for transdermal route. Curr Drug Targets. 2014;15(3):281–91. https://doi.org/10.2174/13894501113146660232.
Waghule T, Singhvi G, Dubey SK, Pandey MM, Gupta G, Singh M et al. Microneedles: a smart approach and increasing potential for transdermal drug delivery system. Elsevier Masson SAS; 2019. p. 1249–58.
Andersen TE, Andersen AJ, Petersen RS, Nielsen LH, Keller SS. Drug loaded biodegradable polymer microneedles fabricated by hot embossing. Microelectron Eng. 2018;195:57–61. https://doi.org/10.1016/j.mee.2018.03.024.
Qiu Y, Li C, Zhang S, Yang G, He M, Gao Y. Systemic delivery of artemether by dissolving microneedles. Int J Pharm. 2016;508(1–2):1–9. https://doi.org/10.1016/j.ijpharm.2016.05.006.
Mc Crudden MTC, Larrañeta E, Clark A, Jarrahian C, Rein-Weston A, Lachau-Durand S, et al. Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension. J Control Release. 2018;292:119–29. https://doi.org/10.1016/j.jconrel.2018.11.002.
Donnelly RF, Larrañeta E. Microarray patches: potentially useful delivery systems for long-acting nanosuspensions. Drug Discovery Today. 2018;23(5):1026–33. https://doi.org/10.1016/j.drudis.2017.10.013.
Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23(5):1008–19. https://doi.org/10.1007/s11095-006-0028-9.
Chu LY, Prausnitz MR. Separable arrowhead microneedles. J Control Release. 2011;149(3):242–9. https://doi.org/10.1016/j.jconrel.2010.10.033.
Lee K, Lee CY, Jung H. Dissolving microneedles for transdermal drug administration prepared by stepwise controlled drawing of maltose. Biomaterials. 2011;32(11):3134–40. https://doi.org/10.1016/j.biomaterials.2011.01.014.
Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA. 2015;112(27):8260–5. https://doi.org/10.1073/pnas.1505405112.
Kochhar JS, Lim WXS, Zou S, Foo WY, Pan J, Kang L. Microneedle integrated transdermal patch for fast onset and sustained delivery of lidocaine. Mol Pharm. 2013;10(11):4272–80. https://doi.org/10.1021/mp400359w.
Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29(13):2113–24. https://doi.org/10.1016/j.biomaterials.2007.12.048.
Wang QL, Zhu DD, Chen Y, Guo XD. A fabrication method of microneedle molds with controlled microstructures. Mater Sci Eng, C. 2016;65:135–42. https://doi.org/10.1016/j.msec.2016.03.097.
Chen MC, Ling MH, Lai KY, Pramudityo E. Chitosan microneedle patches for sustained transdermal delivery of macromolecules. Biomacromol. 2012;13(12):4022–31. https://doi.org/10.1021/bm301293d.
Raja WK, Maccorkle S, Diwan IM, Abdurrob A, Lu J, Omenetto FG, et al. Transdermal delivery devices: Fabrication, mechanics and drug release from silk. Small. 2013;9(21):3704–13. https://doi.org/10.1002/smll.201202075.
Donnelly RF, Majithiya R, Singh TRR, Morrow DIJ, Garland MJ, Demir YK, et al. Design, optimization and characterisation of polymeric microneedle arrays prepared by a novel laser-based micromoulding technique. Pharm Res. 2011;28(1):41–57. https://doi.org/10.1007/s11095-010-0169-8.
Xie L, Zeng H, Sun J, Qian W. Engineering microneedles for therapy and diagnosis: A survey. MDPI AG; 2020.
Zhou J, Ochoa M, Samaddar S, Rahimi R, Badwaik VD, Thompson DH et al., editors. A rapid micro-molding process for fabricating polymeric biodegradable 3D structures using hydrophobic elastomeric molds 2017/02//: Institute of Electrical and Electronics Engineers Inc.
Kim J, Yoon YK, Allen MG. Computer numerical control (CNC) lithography: Light-motion synchronized UV-LED lithography for 3D microfabrication. Journal of Micromechanics and Microengineering. 2016;26(3). https://doi.org/10.1088/0960-1317/26/3/035003.
McGrath MG, Vucen S, Vrdoljak A, Kelly A, O’Mahony C, Crean AM, et al. Production of dissolvable microneedles using an atomised spray process: Effect of microneedle composition on skin penetration. Eur J Pharm Biopharm. 2014;86(2):200–11. https://doi.org/10.1016/j.ejpb.2013.04.023.
Lee K, Jung H. Drawing lithography for microneedles: A review of fundamentals and biomedical applications. Biomaterials; 2012. p. 7309–26.
Vecchione R, Coppola S, Esposito E, Casale C, Vespini V, Grilli S, et al. Electro-drawn drug-loaded biodegradable polymer microneedles as a viable route to hypodermic injection. Adv Func Mater. 2014;24(23):3515–23. https://doi.org/10.1002/adfm.201303679.
Luzuriaga MA, Berry DR, Reagan JC, Smaldone RA, Gassensmith JJ. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Lab Chip. 2018;18(8):1223–30. https://doi.org/10.1039/c8lc00098k.
Goyanes A, Wang J, Buanz A, Martínez-Pacheco R, Telford R, Gaisford S, et al. 3D printing of medicines: engineering novel oral devices with unique design and drug release characteristics. Mol Pharm. 2015;12(11):4077–84. https://doi.org/10.1021/acs.molpharmaceut.5b00510.
Keikhosravi N, Mirdamadian SZ, Varshosaz J, Taheri A. Preparation and characterization of polypills containing aspirin and simvastatin using 3D printing technology for the prevention of cardiovascular diseases. Drug Dev Ind Pharm. 2020;46(10):1665–75. https://doi.org/10.1080/03639045.2020.1820034.
Linares V, Casas M, Caraballo I. Printfills: 3D printed systems combining fused deposition modeling and injection volume filling. Application to colon-specific drug delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2019;134:138–43. https://doi.org/10.1016/j.ejpb.2018.11.021.
Goyanes A, Robles Martinez P, Buanz A, Basit AW, Gaisford S. Effect of geometry on drug release from 3D printed tablets. Int J Pharm. 2015;494(2):657–63. https://doi.org/10.1016/j.ijpharm.2015.04.069.
Homaee Borujeni S, Mirdamadian SZ, Varshosaz J, Taheri A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose. 2020;27(3):1573–89. https://doi.org/10.1007/s10570-019-02881-4.
Lutton REM, Moore J, Larrañeta E, Ligett S, Woolfson AD, Donnelly RF. Microneedle characterisation: the need for universal acceptance criteria and GMP specifications when moving towards commercialisation. Drug Deliv Transl Res. 2015;5(4):313–31. https://doi.org/10.1007/s13346-015-0237-z.
Zhan H, Ma F, Huang Y, Zhang J, Jiang X, Qian Y. Application of composite dissolving microneedles with high drug loading ratio for rapid local anesthesia. Eur J Pharm Sci. 2018;121:330–7. https://doi.org/10.1016/j.ejps.2018.06.014.
Larrañeta E, Moore J, Vicente-Pérez EM, González-Vázquez P, Lutton R, Woolfson AD, et al. A proposed model membrane and test method for microneedle insertion studies. Int J Pharm. 2014;472:65–73. https://doi.org/10.1016/j.ijpharm.2014.05.042.
Ryan E, Garland MJ, Singh TRR, Bambury E, O’Dea J, Migalska K, et al. Microneedle-mediated transdermal bacteriophage delivery. Eur J Pharm Sci. 2012;47:297–304. https://doi.org/10.1016/j.ejps.2012.06.012.
Khan S, Minhas MU, Tekko IA, Donnelly RF, Thakur RRS. Evaluation of microneedles-assisted in situ depot forming poloxamer gels for sustained transdermal drug delivery. Drug Deliv Transl Res. 2019;9:764–82. https://doi.org/10.1007/s13346-019-00617-2.
Larrañeta E, Stewart S, Fallows SJ, Birkhäuer LL, McCrudden MTC, Woolfson AD, et al. A facile system to evaluate in vitro drug release from dissolving microneedle arrays. Int J Pharm. 2016;497:62–9. https://doi.org/10.1016/j.ijpharm.2015.11.038.
Chen J, Huang W, Huang Z, Liu S, Ye Y, Li Q, et al. Fabrication of Tip-Dissolving Microneedles for Transdermal Drug Delivery of Meloxicam. AAPS PharmSciTech. 2018;19:1141–51. https://doi.org/10.1208/s12249-017-0926-7.
Khoee S, Hossainzadeh MT. Effect of O/S/W process parameters on 17β-EV loaded nanoparticles properties. Colloids Surf, B. 2010;75(1):133–40. https://doi.org/10.1016/j.colsurfb.2009.08.021.
O'Mahony C, Bocchino A, Haslinger MJ, Brandstatter S, Außerhuber H, Schossleitner K et al. Piezoelectric inkjet coating of injection moulded, reservoir-tipped microneedle arrays for transdermal delivery. Journal of Micromechanics and Microengineering. 2019;29(8). https://doi.org/10.1088/1361-6439/ab222b.
Cicinelli E, Savino F, Cagnazzo I, Scorcia P, Galantino P. Progesterone administration by nasal spray in menopausal women: Comparison between two different spray formulations. Gynecol Endocrinol. 1992;6(4):247–51. https://doi.org/10.3109/09513599209024986.
Baloš S, Pili B, Petronijevi B, Markovi D, Mirkovi S, Šarev I. Improving mechanical properties of flowable dental composite resin by adding silica nanoparticles Poboljšanje mehanikih svojstava tenog kompozita dodavanjem nanoestica silicijum-dioksida. Vojnosanit Pregl. 2013;70(5):477–83. https://doi.org/10.2298/VSP1305477B.
Su B, Zhou Y-G, Wu H-H. Influence of mechanical properties of polypropylene/low-density polyethylene nanocomposites. Nanomaterials and Nanotechnology. 2017;7:184798041771592-. https://doi.org/10.1177/1847980417715929.
Pan J, Ruan W, Qin M, Long Y, Wan T, Yu K, et al. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci Rep. 2018;8(1):1–1. https://doi.org/10.1038/s41598-018-19463-2.
Machekposhti S, Soltani M, Najafizadeh P, Ebrahimi SA, Chen P. Biocompatible polymer microneedle for topical/dermal delivery of tranexamic acid. J Control Release. 2017;261:87–92. https://doi.org/10.1016/j.jconrel.2017.06.016.
Park JH, Prausnitz MR. Analysis of the mechanical failure of polymer microneedles by axial force. J Korean Phys Soc. 2010;56:1223–7. https://doi.org/10.3938/jkps.56.1223.
Arafat B, Wojsz M, Isreb A, Forbes RT, Isreb M, Ahmed W, et al. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur J Pharm Sci. 2018;118:191–9. https://doi.org/10.1016/j.ejps.2018.03.019.
Tan DK, Maniruzzaman M, Nokhodchi A. development and optimisation of novel polymeric compositions for sustained release theophylline caplets (PrintCap) via FDM 3D printing. Polymers. 2019;12(1):27-. https://doi.org/10.3390/polym12010027.
Joshi BV, Patil VB, Pokharkar VB. Compatibility studies between carbamazepine and tablet excipients using thermal and non-thermal methods. Drug Dev Ind Pharm. 2002;28(6):687–94. https://doi.org/10.1081/DDC-120003860.
Botha SA, Lötter AP. Compatibility study between naproxen and tablet excipients using differential scanning calorimetry. Drug Dev Ind Pharm. 1990;16(4):673–83. https://doi.org/10.3109/03639049009104410.
Pineda-Hernández MT, Pérez-Urizar JT, Ganem-Rondero A. Thermo-reversible in situ forming implant with nanostructured lipid carriers (NLC) as a delivery system for the administration of estradiol valerate. Drug Deliv Transl Res. 2020;10(5):1393–402. https://doi.org/10.1007/s13346-019-00704-4.
Yang TC, Yeh CH. Morphology and mechanical properties of 3D printed wood fiber/polylactic acid composite parts using Fused Deposition Modeling (FDM): The effects of printing speed. Polymers. 2020;12(6):1334-. https://doi.org/10.3390/POLYM12061334.
Spinelli G, Kotsilkova R, Ivanov E, Petrova-Doycheva I, Menseidov D, Georgiev V et al. Effects of filament extrusion, 3D printing and hot-pressing on electrical and tensile properties of poly(lactic) acid composites filled with carbon nanotubes and graphene. Nanomaterials. 2019;10(1):35-. https://doi.org/10.3390/nano10010035.
Kim H, Lee S. Characterization of electrical heating of graphene/PLA honeycomb structure composite manufactured by CFDM 3D printer. Fashion and Textiles. 2020;7(1). https://doi.org/10.1186/s40691-020-0204-2.
Akhayere E, Kavaz D, Vaseashta A. Synthesizing nano silica nanoparticles from barley grain waste: Effect of temperature on mechanical properties. Polish Journal of Environmental Studies. 2019;28(4):2513–21. https://doi.org/10.15244/pjoes/91078.
Cavallari C, Fini A, Ceschel G. Design of olanzapine/lutrol solid dispersions of improved stability and performances. Pharmaceutics. 2013;5(4):570–90. https://doi.org/10.3390/pharmaceutics5040570.
Pop MA, Croitoru C, Bedő T, Geamăn V, Radomir I, Cosnită M, et al. Structural changes during 3D printing of bioderived and synthetic thermoplastic materials. J Appl Polym Sci. 2019;136(17):47382. https://doi.org/10.1002/app.47382.
Venkataram S, Khohlokwane M, Wallis SH. Differential scanning calorimetry as a quick scanning technique for solid state stability studies. Drug Dev Ind Pharm. 1995;21(7):847–55. https://doi.org/10.3109/03639049509026649.
Rao M, Ranpise A, Borate S, Thanki K. Mechanistic evaluation of the effect of sintering on Compritol® 888 ATO matrices. AAPS PharmSciTech. 2009;10(2):355–60. https://doi.org/10.1208/s12249-009-9211-8.
Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: Fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104:51–66. https://doi.org/10.1016/j.jconrel.2005.02.002.
Park JH, Yoon YK, Choi SO, Prausnitz MR, Allen MG. Tapered conical polymer microneedles fabricated using an integrated lens technique for transdermal drug delivery. IEEE Trans Biomed Eng. 2007;54:903–13. https://doi.org/10.1109/TBME.2006.889173.
Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J Biomech. 2004;37:1155–63. https://doi.org/10.1016/j.jbiomech.2003.12.010.
Moronkeji K, Todd S, Dawidowska I, Barrett SD, Akhtar R. The role of subcutaneous tissue stiffness on microneedle performance in a representative in vitro model of skin. J Control Release. 2017;265:102–12. https://doi.org/10.1016/j.jconrel.2016.11.004.
Neupane R, Boddu SHS, Renukuntla J, Babu RJ, Tiwari AK. Alternatives to biological skin in permeation studies: Current trends and possibilities. Pharmaceutics. 2020;12(2):152. https://doi.org/10.3390/pharmaceutics12020152.
Bravo D, Rigley TH, Gibran N, Strong DM, Newman-Gage H. Effect of storage and preservation methods on viability in transplantable human skin allografts. Burns. 2000;26:367–78. https://doi.org/10.1016/S0305-4179(99)00169-2.
Yavuz B, Chambre L, Harrington K, Kluge J, Valenti L, Kaplan DL. Silk fibroin microneedle patches for the sustained release of levonorgestrel. ACS Appl Bio Mater. 2020;3:5375–82. https://doi.org/10.1021/acsabm.0c00671.
Wischke C, Schwendeman SP. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int J Pharm. 2008;364(2):298–327. https://doi.org/10.1016/j.ijpharm.2008.04.042.
Farah S, Anderson DG, Langer R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv Drug Deliv Rev. 2016;107:367–92. https://doi.org/10.1016/j.addr.2016.06.012.
Weir NA, Buchanan FJ, Orr JF, Dickson GR. Degradation of poly-L-lactide. Part 1: In vitro and in vivo physiological temperature degradation. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2004;218:307–19. https://doi.org/10.1243/0954411041932782.
Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv. 2010;7(4):429–44. https://doi.org/10.1517/17425241003602259.
Yuan Y, Choi K, Choi SO, Kim J. Early stage release control of an anticancer drug by drug-polymer miscibility in a hydrophobic fiber-based drug delivery system. RSC Adv. 2018;8:19791–803. https://doi.org/10.1039/c8ra01467a.
Singh M, Jonnalagadda S. Design and characterization of 3D printed, neomycin-eluting poly-L-lactide mats for wound-healing applications. J Mater Sci - Mater Med. 2021;32:44. https://doi.org/10.1007/s10856-021-06509-7.
Dinarvand R, Moghadam SH, Sayar P, Alaee M, Atyabi F. Preparation of a polymeric reservoir naltrexone delivery device: effect of PEG content of the PLA membrane on drug release. Therapy. 2005;2:407–13. https://doi.org/10.2217/14750708.2.3.407.
Funding
This study was supported by a grant (No. 198107) from Isfahan University of Medical Sciences, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The animal study was approved by the Medical Ethics Committee at the Isfahan University of Medical Sciences (Ethics code: IR.MUI.RESEARCH.REC.1398.358).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khosraviboroujeni, A., Mirdamadian, S.Z., Minaiyan, M. et al. Preparation and characterization of 3D printed PLA microneedle arrays for prolonged transdermal drug delivery of estradiol valerate. Drug Deliv. and Transl. Res. 12, 1195–1208 (2022). https://doi.org/10.1007/s13346-021-01006-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01006-4