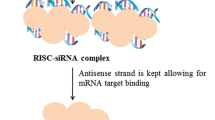
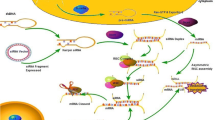
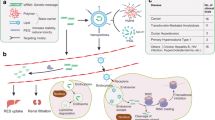
Abstract
Along with the evolutionary breakthrough of RNA interference and the applicability for gene knockdown, a subsequent development in siRNA-based therapeutics has been attained. The gene therapy based on RNAi is in transition progress from the research aspects to clinical base. Being a potent tool, siRNA is used as therapeutic against several disorders. Cancer which is one of the deadliest diseases is now treated with an advanced mechanism of siRNA delivery inside the genome, leading to gene silencing; thereby, blocking translation of gene to form protein. siRNA tool delivers remedial effects with the advantages of safe delivery and efficiency. Despite its merits, barriers including instability at physiological conditions, lack of ability to cross biological membranes, off-targets, and safety are also associated with siRNA delivery system. The gene silencing efficiency values both in vitro and in vivo reported in the past years have been reviewed by material type (lipid, polymer, silica, porous silicon, and metal). This review presents a deep insight in the development of targeted delivery of siRNA. Since several clinical trials have also been performed regarding the siRNA delivery against cancer, it can also be stated that the delivery system should be good enough to achieve effective siRNA drug development.
Graphical abstract







Similar content being viewed by others
References
World Health Organization. International Agency for Research on Cancer (IARC). 2018.
Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–37.
Wheeler DA, Wang L. From human genome to cancer genome: the first decade. Genome Res. 2013;23(7):1054–62.
Tong WY, Alnakhli M, Bhardwaj R, Sinoula A, Sougata S, Fraser C, Kuche T, Kuss B, Voelcker NH. Delivery of siRNA in vitro and in vivo using PEI-capped porous silicon nanoparticles to silence MRP1 and inhibit proliferation in glioblastoma. J Nanobiotechnology. 2018;16(1):38.
Artiga Á, Serrano-Sevilla I, De Matteis L, Mitchell SG, de la Fuente JM. Current status and future perspectives of gold nanoparticle vectors for siRNA delivery. J Mater Chem B. 2019;7(6):876–96.
Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–70.
Kim B, Park JH, Sailor MJ. Rekindling RNAi therapy: materials design requirements for in vivo siRNA delivery. Advance Materials. 2019;1903637:1–23.
Cong F, Jun W. Delivery systems for siRNA drug development in cancer therapy. Asian J Pharm Sci. 2014;10:1–12.
Mahmoodi Chalbatani G, Dana H, Gharagouzloo E, Grijalvo S, Eritja R, Logsdon CD, Memari F, Miri SR, Rad MR, Marmari V. Small interfering RNAs (siRNAs) in cancer therapy: a nano-based approach. Int J Nanomedicine. 2019;14:3111–28.
Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, Hartmann G. Sequence-specific potent induction of IFN-aby short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–70.
Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9:57–67.
Marques JT, Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399–405.
Riley RS, Dang MS, Billingsley MM, Abraham B, Gundlach L, Day ES. Evaluating the mechanisms of light-triggered siRNA release from nanoshells for temporal control over gene regulation. Nano Lett. 2018;18:3565.
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9.
Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95.
Bumcrot D, Manoharan M, Koteliansky V, Sah DW. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2:711–9.
Dominska M, Dykxhoorn DM. Breaking down the barriers siRNA delivery and endosome escape. J Cell Sci. 2010;123:1183–9.
Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and bio-distribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–15.
Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond). 2016;11(6):673–92.
Van de Water FM, Boerman OC, Wouterse AC, Peters JG, Russel FG, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug Metab Dispos. 2006;34:1393–7.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69.
Larson SD, Jackson LN, Chen LA, Rychahou PG, Evers BM. Effectiveness of siRNA uptake in target tissues by various delivery methods. Surgery. 2007;142:262–9.
Schultz N, Marenstein DR, De Angelis DA, Wang WQ, Nelander S, Jacobsen A, Marks DS, Massague J, Sander C. Off-target effects dominate a large-scale RNAi screen for modulators of the TGF-pathway and reveal microRNA regulation ofTGFBR2. Silence. 2011;2:1–20.
Wang Z, Li S, Zhang M, Ma Y, Liu Y, Gao W, Zhang J, Gu Y. Laser-triggered small interfering RNA releasing gold nanoshells against heat shock protein for sensitized photothermal therapy. Adv Sci (Weinh). 2016;4(2):1600327.
Conde J, Ambrosone A, Sanz V, Hernandez Y, Marchesano V, Tian F, Child H, Berry CC, Ibarra MR, Baptista PV, Tortiglione C, de la Fuente JM. Design of multifunctional gold nanoparticles for in vitro and in vivo gene silencing. ACS Nano. 2012;6:8316–24.
Mahajan UM, Teller S, Sendler M, Palankar R, Van den Brandt C, Schwaiger T, Kuhn JP, Ribback S, Glockl G, Evert M, Weitschies W, Hosten N, Dombrowski F, Delcea M, Weiss FU, Lerch MM, Mayerle J. Tumour specific delivery of siRNA-coupled superparamagnetic iron oxide nanoparticles, targeted against PLK1, stops progression of pancreatic cancer. Gut. 2016;65:1838–49.
Scherr M, Battmer K, Winkler T, Heidenreich O, Ganser A, Eder M. Specific inhibition of her abl gene expression by small interfering RNA. Blood 2003;101:1566–1569.
Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–7.
Choudhury A, Charo J, Parapuram SK, Hunt RC, Hunt DM, Seliger B, Kiessling R. Small interfering RNA (siRNA) inhibits the expression of the Her2/neu gene, up-regulates HLA class 1 and induces apoptosis of HER2/neu positive tumor cell lines. Int J Cancer. 2004;108: 71–7.
Naito Y, Yoshimura J, Morishita S, Ui-Tei K. siDirect 2.0:updated software for designing functional siRNA with reduced seed-dependent off-target effect. BioMed Central 2009;10–392.
Luck S, Kreszies T, Strickert M, Schweizer Kuhlmann M, Douchkov D. siRNA-Finder (si-Fi) Software for RNAi-target design and off-target prediction. Front Plant Sci. 2019;10:1–12.
Qie Y, Yuan H, Von Roemeling CA, Chen Y, Liu X, Shih KD, Knight JA, Tun HW, Wharen RE, Jiang W, Kim BYS. Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes Sci Rep. 2016;6:26269.
Nogawa M, Yuasa T, Kimura S, Tanaka M, Kuroda J, Sato K, Yokota A, Segawa H, Toda Y, Kageyama S, Yoshiki T, Okada Y, Maekawa T. Intravesical administration of small interfering RNA targeting PLK-1 successfully prevents the growth of bladder cancer. J Clin Invest. 2005;115:978–85.
Song E, Zhu P, Lee SK, Choudhary D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol. 2005;23:709–17.
Kaddah S, Khreich N, Kaddah F, Charcosset C, Greige-Gerges H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem Toxicol. 2018;113:40–8.
Pinnapireddy SR, Duse L, Strehlow B, Schäfer J, Bakowsky U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids Surf B Biointerfaces. 2017;158:93–101.
Whitehead KA, Langer R, Anderson DG. Knocking downbarriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–38.
Deleavey GF, Damha MJ. Designing chemically modified oligonucleotides for targeted gene silencing. Chem Biol. 2012;19:937–54.
Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–98.
Malek A, Merkel O, Fink L, Czudbayko F, Kissel T, Aigner A. In vivo pharmacokinetics, tissue distribution and underlying mechanisms of various PEI (-PEG)/siRNA complexes. Toxicol Appl Toxicol. 2009;236:97–108.
Akinc A, Querbes W, De S, Qin J, Frank-Kamenetsky M, Jayaprakash KN, Jayaraman M, Rajeev KG, Cantley WL, Dorkin JR, Butler, JS, Qin L, Racie T, Sprague A, Fava E, Zeigerer A, Hope MJ, Zerial M, Sah, DW, Fitzgerald K, Tracy MA, Manoharan M, Koteliansky V, Fougerolles Ad, Maier MA. Targeted delivery of RNAitherapeutics with endogenous and exogenous ligand-basedmechanisms. Mol Ther 2010;18:1357–1364.
Wang D, Lin J, Jia F, Tan X, Wang Y, Sun X, Cao X, Che F, Lu H, Gao X, Shimkonis JC, Nyoni Z, Lu X, Zhang K. Bottlebrush-architectured poly(ethylene glycol) as an efficient vector for RNA interference in vivo. Sci Adv. 2019;5(2):9322.
Jarad G, Miner JH. Update on the glomerular filtration barrier. Curr Opin Nephrol Hypertens. 2009;18:226.
Lee H, Lytton-Jean AKR, Chen Y, Love KT, Park AI, Karagiannis ED, Sehgal A, Querbes W, Zurenko CS, Jayaraman M, Peng CG, Charisse K, Borodovsky A, Manoharan M, Donahoe JS, Truelove J, Nahrendorf M, Langer R, Anderson DG. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivosiRNA delivery. Nat Nano technol. 2012;7:389–93.
Rozema DB, Lewis DL, Wakefield DH, Wong SC, Clein JJ, Roesch PL, Bertin SL, Reppen TW, Chu Q, Blokhin AV, Hagstrom JE, Wolff JA. DynamicPolyConjugates for targeted in vivo delivery of siRNA tohepatocytes. Proceddings of National Academy of Science. 2007;104:12982–7.
Yu B, Zhao X, Lee LJ, Lee RJ. Targeted delivery systems for oligonucleotide therapeutics. AAPS J. 2009;11:195–203.
Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. NatNanotechnol. 2013;8:137–43.
Bolhassani A. Potential efficacy of cell-penetrating peptides for nucleic acid and drug delivery in cancer. Biochim Biophys Acta-Rev Cancer. 2011;1816:232–46.
Bitko V, Musiyenko A, Shulyayeva O, Barik S. Inhibition of respiratory viruses by nasally administered siRNA. Nature Med. 2005;11:50–5.
Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Narayanannair Jayaprakash K, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DW, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nature Biotech. 2008;26:561–569.
Grzelinski M, Urban-Klein B, Martens T, Lamszus K, Bakowsky U, Höbel S, Czubayko F, Aigner A. RNA interference-mediated gene silencing of pleiotrophin through polyethyleniminecomplexed small interfering RNAs in vivo exerts antitumoral effects in glioblastoma xenografts. Hum. Gene Ther. 2006;17:751–766.
Wang Y, Li Z, Han Y, Liang LH, Ji A. Nanoparticle-based delivery system for application of siRNA in vivo. CurrentDrug Metabolism. 2010;11(2):182–96.
Dande P, Prakash TP, Sioufi N, Gaus H, Jarres R, Berdeja A, Swayze EE, Griffey RH, Bhat B. Improving RNA interference in mammalian cells by 40-thio-modified small interfering RNA (siRNA): effect on siRNA activity and nuclease stability when used in combination with 20-O-alkyl modifications. J Med Chem. 2006;49:1624–34.
Hall AH, Wan J, Shaughnessy EE, Ramsay Shaw B, Alexander KA. RNA interference using borano phosphate siRNAs: structure-activity relationships. Nucleic Acids Res. 2004;32:5991–6000.
Miller A, Tanner J. Essentials of chemical biology: structure and dynamics of biological macromolecules. Chichester: John Wiley & Sons; 2008.
Turanek J, Miller AD, Kauerova Z, Lukac R, Masek J, Koudelka S, Raska M. Chapter 4: Lipid Based Nanoparticles and Microbubbles-Multifunctional Lipid-Based Biocompatible Particles for in vivo Imaging and Theranostics. Advances in Bio Engineering Intech 2015;79–116.
Miller AD. Delivery of RNAi therapeutics: work in progress. Expert Rev Med Devices. 2013;10(6):781–811.
Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5(1):25–44.
Wu SY, McMillan NAJ. Lipidic systems for in vivo siRNA delivery. AAPS Journal. 2009;11(4):639–52.
Landen CN Jr, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Can Res. 2005;65(15):6910–8.
Halder J, Kamat AA, Landen CN Jr, Han LY, Lutgendorf SA, Lin YG, Merritt WM, Jennings NB, Chavez-Reyes A, Coleman RL, Gershenson DM, Schmandt R, Cole SW, Lopez-Berestein G, Sood AK. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12(16):4916–24.
NCI Update. EphA2 siRNA in Treating Patients with advanced or recurrent solid tumors. 2012. http://www.clinicaltrials.gov/ct2/show/NCT01591356.
Ozpolat B, Sood AK, Lopez-Berestein G. Nanomedicine-based approaches for the delivery of siRNA in cancer. J Intern Med. 2010;267(1):44–53.
Taetz S, Bochot A, Surace C, Arpicco S, Renoir JM, Schaefer UF, Marsaud V, Kerdine-Roemer S, Lehr CM, Fattal E. Hyaluronic acid-modified DOTAP/DOPE liposomes for the targeted delivery of anti-telomerase siRNA to CD44-expressing lung cancer cells. Oligonucleotides. 2009;19(2):103–16.
Ewe A, Panchal O, Pinnapireddy SR, Bakowsky U, Przybylski S, Temme A, Aigner A. Liposome-polyethylenimine complexes (DPPC-PEI lipopolyplexes) for therapeutic siRNA delivery in vivo. Nanomedicine. 2017;13(1):209–18.
Jin J, Bae KH, Yang H, Lee SJ, Kim H, Kim Y, Juu KM, Seo SW, Park TG, Nam DH. In vivo specific delivery of c-MetsiRNA to glioblastoma using cationic solid lipid nanoparticles. Bioconjug Chem. 2011;22(12):2568–72.
Jeffs LB, Palmer LR, Ambegia EG, Giesbrecht C, Ewanick S, MacLachlan I. A scalable, extrusion-free method for efficient liposomal encapsulation of plasmid DNA. Pharm Res. 2005;22(3):362–72.
Kim B, Sun S, Varner JA, Howell SB, Ruoslahti E, Sailor MJ. Securing the payload, finding the cell, and avoiding the endosome: peptide-targeted, fusogenic porous silicon nanoparticles for delivery of siRNA. Adv Mater. 2019;31(35):1902952.
Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, Ambegia E, McClintok K, MacLachlan I. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119:661.
Wang X, Wang Y, Chen ZG, Shin DM. Advances of cancer therapy by nanotechnology. Cancer Res Treat. 2009;41:1–11.
Miele E, Spinelli GP, Miele E, Tomao F, Tamao S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int J Nanomed. 2009;4:99–105.
Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, Lin KP, Vita G, Attarian S, Plante-Bordeneuve V, Mezei MM, Campistol JM, Buades J, Brannagan TH 3rd, Kim BJ, Oh J, Parman Y, Sekijima Y, Hawkins PN, Solomon SD, Polydefkis M, Dyck PJ, Gandhi PJ, Goyal S, Chen J, Strahs AL, Nochur SV, Sweetser MT, Garg PP, Vaishnaw AK, Gollob JA, Suhr OB. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21.
Nair JK, Attarwala H, Sehgal A, Wang Q, Aluri K, Zhang X, Gao M, Liu J, Indrakanti R, Schofield S, Kretschmer P, Brown CR, Gupta S, Willoughby JLS, Boshar JA, Jadhav V, Charisse K, Zimmermann T, Fitzgerald K, Manoharan M, Rajeev KG, Akinc A, Hutabarat R, Maier MA. Impact of enhanced metabolic stability on pharmacokinetics and pharmacody- namics of GalNAc-siRNA conjugates. Nucleic Acids Res. 2017;45(19):10969–77.
Foster DJ, Brown CR, Shaikh S, Trapp C, Schlegel MK, Qian K, Sehgal A, Rajeev KG, Jadhav V, Manoharan M, Kuchimanchi S, Maier MA, Milstein S. Advanced siRNA designs further improve in vivo performance of GalNAc-siRNA conjugates. Mol Ther. 2018;26(3):708–17.
Chen C, Posocco P, Liu X, Cheng Q, Laurini E, Zhou J, Liu C, Wang Y, Tang J, Col VD, Yu T, Giorgio S, Fermeglia M, Qu F, Liang Z, Rossi JJ, Liu M, Rocchi P, Pricl S, Peng L. Mastering dendrimer self-assembly for efficient siRNA delivery: from conceptual design to in vivo efficient gene silencing. Small. 2016;12(27):3667–76.
Feldmann DP, Cheng Y, Kandil R, Xie Y, Mohammadi M, Harz H, Sharma A, Peeler DJ, Moszczynska A, Leonhardt H, Pun SH, Merkel OM. In vitro and in vivo delivery of siRNA via VIPER polymer system to lung cells. J Controlled Release. 2018;276:50–8.
Navarro G, Sawant RR, Biswas S, Essex S. Tros de Ilarduya C, Torchilin VPP-glycoprotein silencing with siRNA delivered by DOPE-modified PEIover comes doxorubicin resistance in breast cancer cells. Nano medicine. 2012;7:65–78.
Sun TM, Du JZ, Yan LF, Mao HQ, Wang J. Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials. 2008;29:4348–55.
Jilek S, Merkle HP, Walter E. DNA-loaded biodegradable microparticles as vaccine delivery systems and their interaction with dendritic cells. Adv Drug Deliv Rev. 2005;57:377–90.
Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–33.
Yang XZ, Dou S, Sun TM, Mao CQ, Wang HX, Wang J. Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy. J Control Release. 2011;156:203–11.
Wu C, Li J, Wang W, Hammond PT. Rationally designed polycationic carriers for potent polymeric siRNA-mediated gene silencing. ACS Nano. 2018;12(7):6504–14.
Jeong JH, Mok H, Oh YK, Park TG. siRNA conjugate delivery systems. Bioconjug Chem. 2008;20:5–14.
Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, Zimmermann T, Koteliansky V, Manoharan M, Stoffel M. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–57.
Alzhrani R, Alsaab HO, Petrovici A, Bhise K, Vanamala K, Sau S, Krinock MJ, Iyer AK. Improving the therapeutic efficiency of noncoding RNAs in cancers using targeted drug delivery systems. Drug Discov Today. 2020;25(4):718–30.
Chiu YL, Ali A, Chu C, Cao H, Rana TM. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Biol. 2004;11:1165–75.
Moschos SA, Jones SW, Perry MM, Williams AE, Erjefalt JS, Turner JJ, Barnes PJ, Sproat BS, Gait MJ, Lindsay MA. Lung delivery studies using siRNA conjugated to TAT (48–60) and penetrate in reveal peptide induced reduction in gene expression and induction of innate immunity. BioconjugChem. 2007;18:1450–9.
Cesarone G, Edupuganti OP, Chen CP, Wickstrom E. Insulin receptor substrate 1 knockdown in human MCF7 ERþ breast cancer cells by nuclease-resistant IRS1 siRNA conjugated to a disulfide-bridged D-peptide analogue of insulin-like growth factor 1. Bioconjug Chem. 2007;18:1831–40.
Xia CF, Zhang Y, Zhang Y, Boado RJ, Pardridge WM. Intravenous siRNA of brain cancer with receptor targeting and avidin biotin technology. Pharm Res. 2007;24:2309–16.
Chu TC, Twu KY, Ellington AD, Levi M. Aptamer mediated siRNA delivery. Nucleic Acids Res. 2006;34:73.
Shen S, Mao CQ, Yang XZ, Du XJ, Liu Y, Zhu YH, Wang J. Cationic lipid-assisted polymeric nanoparticles-mediated GATA2 siRNA delivery for synthetic lethal therapy of KRAS mutant non-small-cell lung carcinoma. Mol Pharm. 2014;11:2612–22.
Hori S, Herrera A, Rossi JJ, Zhou J. Current advances in aptamers for cancer diagnosis and therapy. Cancers. 2018;10(1):9.
Greening DW, Xu R, Gopal SK, Rai A, Simpson RJ. Proteomic insights into extracellular vesicle biology - defining exosomes and shed microvesicles. Expert Rev Proteomics. 2017;14(1):69–95.
Montecalvo A, Larregina AT, Shufesky WJ, Stolz DB, Sullivan MLG, Karlsson JM, Baty CJ, Gibson GA, Erdos G, Wang Z, Milosevic J, Tkacheva OA, Divito SJ, Jordan R, Lyons-Weiler J, Watkins SC, Morelli AE. Mechanismof transfer of functional microRNAs between mousedendritic cells via exosomes. Blood. 2012;119:756–66.
William H, Zhang XQ, Xu X. Biomaterials in siRNA Delivery: A Comprehensive Review. Wiley Online Library. 2016.
De Fougerolles A, Novobrantseva T. siRNA and the lung: research tool or therapeutic drug? Curr Opin Pharmacol. 2008;8:280–5.
Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Pharmaceutical research. 2015;32(6).
Acknowledgements
Authors thank to Shri Guru Ram Rai University, Patel Nagar, Dehradun, Uttarakhand, India, for providing high speed Internet connectivity.
Author information
Authors and Affiliations
Contributions
Indra Rautela and Aditi Sharma carried information retrieval and compilation; Pallavi Dheer, Priya Thapliyal, and Shweta Sahni made first draft of the manuscript; Vimlendu Bhushan Sinha critically reviewed the manuscript and finalized the manuscript in current form; Manish Dev Sharma conceptualized, supervised, and finalized the manuscript for publication.
Corresponding author
Ethics declarations
Consent for publication
Taking from all the authors.
Conflict interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rautela, I., Sharma, A., Dheer, P. et al. Extension in the approaches to treat cancer through siRNA system: a beacon of hope in cancer therapy. Drug Deliv. and Transl. Res. 12, 1002–1016 (2022). https://doi.org/10.1007/s13346-021-00995-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00995-6