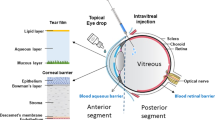
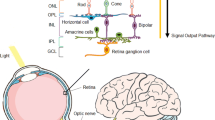
Abstract
The retinal physiology can accrue oxidative damage and inflammatory insults due to age and metabolic irregularities. Two notable diseases that involve retinal and choroidal neovascularization are proliferative diabetic retinopathy and wet age-related macular degeneration. Currently, these diseases are mainly treated with anti-VEGF drugs (VEGF = vascular endothelial growth factor), generally on a monthly dosage scheme. We discuss recent developments for the treatment of these diseases, including bioactive tissue-engineered materials, which may reduce frequency of dosage and propose a path forward for improving patient outcomes.

Development of materials for long-term intravitreal delivery for management of posterior segment diseases




Similar content being viewed by others
References
Yaqoob Z, Wu J, Yang C. Spectral domain optical coherence tomography: a better OCT imaging strategy. Biotechniques. 2005;39:S6–13.
Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–17.
Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44:260–77.
Viets K, Eldred K, Johnston RJ Jr. Mechanisms of photoreceptor patterning in vertebrates and invertebrates. Trends Genet. 2016;32:638–59.
Wong TY, Cheung CM, Larsen M, Sharma S, Simo R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012.
van Lookeren Campagne M, LeCouter J, Yaspan BL, Ye W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol. 2014;232:151–64.
Chrzanowska M, Modrzejewska A, Modrzejewska M. New insight into the role of the complement in the most common types of retinopathy-current literature review. Int J Ophthalmol. 2018;11:1856–64.
SpringerLink (Online service); Springer International: Berlin, etc.; 1982, p v.
American Diabetes Association 2017; Vol. 2017.
Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649–56.
Hammes HP, Lin J, Bretzel RG, Brownlee M, Breier G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes. 1998;47:401–6.
Nentwich MM, Ulbig MW. Diabetic retinopathy - ocular complications of diabetes mellitus. World J Diabetes. 2015;6:489–99.
Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
Simo R, Carrasco E, Garcia-Ramirez M, Hernandez C. Angiogenic and antiangiogenic factors in proliferative diabetic retinopathy. Curr Diabetes Rev. 2006;2:71–98.
Querques G, Delle Noci N. Proinflammatory cytokines and angiogenic and antiangiogenic factors in vitreous of patients with proliferative diabetic retinopathy and Eales' disease (ED). Retina. 2009;29:121–3 author reply 123.
Stem MS, Gardner TW. Neurodegeneration in the pathogenesis of diabetic retinopathy: molecular mechanisms and therapeutic implications. Curr Med Chem. 2013;20:3241–50.
Feenstra DJ, Yego EC, Mohr S. Modes of retinal cell death in diabetic retinopathy. J Clin Exp Ophthalmol. 2013;4:298.
Kadlubowska J, Malaguarnera L, Waz P, Zorena K. Neurodegeneration and neuroinflammation in diabetic retinopathy: potential approaches to delay neuronal loss. Curr Neuropharmacol. 2016;14:831–9.
Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–8.
Yoshimura T, Sonoda KH, Sugahara M, Mochizuki Y, Enaida H, Oshima Y, et al. Comprehensive analysis of inflammatory immune mediators in vitreoretinal diseases. PLoS One. 2009;4:e8158.
Adamiec-Mroczek J, Oficjalska-Mlynczak J, Misiuk-Hojlo M. Roles of endothelin-1 and selected proinflammatory cytokines in the pathogenesis of proliferative diabetic retinopathy: analysis of vitreous samples. Cytokine. 2010;49:269–74.
Liu Y, Biarnes Costa M, Gerhardinger C. IL-1beta is upregulated in the diabetic retina and retinal vessels: cell-specific effect of high glucose and IL-1beta autostimulation. PLoS One. 2012;7:e36949.
Aghdam SY, Gurel Z, Ghaffarieh A, Sorenson CM, Sheibani N. High glucose and diabetes modulate cellular proteasome function: implications in the pathogenesis of diabetes complications. Biochem Biophys Res Commun. 2013;432:339–44.
Xu HZ, Le YZ. Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci. 2011;52:2160–4.
Cunha-Vaz J, Bernardes R, Lobo C. Blood-retinal barrier. Eur J Ophthalmol. 2011;21(Suppl 6):S3–9.
Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48.
Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26:2653–64.
Lang GE. Diabetic macular edema. Ophthalmologica. 2012;227(Suppl 1):21–9.
Petropoulos IK, Koliopoulos JX. Severe proliferative diabetic retinopathy. N Engl J Med. 2007;356:1979.
Laouri M, Chen E, Looman M, Gallagher M. The burden of disease of retinal vein occlusion: review of the literature. Eye (Lond). 2011;25:981–8.
Brand CS. Management of retinal vascular diseases: a patient-centric approach. Eye (Lond). 2012;26(Suppl 2):S1–16.
Cummings M, Cunha-Vaz J. Treatment of neovascular age-related macular degeneration in patients with diabetes. Clin Ophthalmol. 2008;2:369–75.
Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, et al. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimers Dement. 2014;10:135–42.
Feke GT, Hyman BT, Stern RA, Pasquale LR. Retinal blood flow in mild cognitive impairment and Alzheimer's disease. Alzheimers Dement (Amst). 2015;1:144–51.
Ohno-Matsui K. Parallel findings in age-related macular degeneration and Alzheimer's disease. Prog Retin Eye Res. 2011;30:217–38.
Keenan TD, Goldacre R, Goldacre MJ. Associations between age-related macular degeneration, Alzheimer disease, and dementia: record linkage study of hospital admissions. JAMA Ophthalmol. 2014;132:63–8.
Snyder PJ, Johnson LN, Lim YY, Santos CY, Alber J, Maruff P, et al. Nonvascular retinal imaging markers of preclinical Alzheimer's disease. Alzheimers Dement (Amst). 2016;4:169–78.
Ward ME, Chen R, Huang HY, Ludwig C, Telpoukhovskaia M, Taubes A, et al. Individuals with progranulin haploinsufficiency exhibit features of neuronal ceroid lipofuscinosis. Sci Transl Med. 2017;9.
Mukherjee N, McBurney-Lin S, Kuo A, Bedlack R, Tseng H. Retinal thinning in amyotrophic lateral sclerosis patients without ophthalmic disease. PLoS One. 2017;12:e0185242.
Seagle BL, Rezai KA, Kobori Y, Gasyna EM, Rezaei KA, Norris JR Jr. Melanin photoprotection in the human retinal pigment epithelium and its correlation with light-induced cell apoptosis. Proc Natl Acad Sci U S A. 2005;102:8978–83.
Dowler JG. Laser management of diabetic retinopathy. J R Soc Med. 2003;96:277–9.
Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB 3rd, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681–6.
Silva PS, Sun JK, Aiello LP. Role of steroids in the management of diabetic macular edema and proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24:93–9.
Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(1695):e1691–15.
Mason JO 3rd, Nixon PA, White MF. Intravitreal injection of bevacizumab (Avastin) as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2006;142:685–8.
Gonzalez VH, Giuliari GP, Banda RM, Guel DA. Intravitreal injection of pegaptanib sodium for proliferative diabetic retinopathy. Br J Ophthalmol. 2009;93:1474–8.
Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL 3rd, Friedman SM, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609–14.
Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, Antoszyk AN, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–203.
Ng EW, Shima DT, Calias P, Cunningham ET Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat Rev Drug Discov. 2006;5:123–32.
Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145–52.
Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389:2193–203.
Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR, et al. Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmol. 2016;134:888–96.
Jonas JB. Intravitreal triamcinolone acetonide for diabetic retinopathy. Dev Ophthalmol. 2007;39:96–110.
Takamura Y, Shimura M, Katome T, Someya H, Sugimoto M, Hirano T, et al. Effect of intravitreal triamcinolone acetonide injection at the end of vitrectomy for vitreous haemorrhage related to proliferative diabetic retinopathy. Br J Ophthalmol. 2018.
Dugel PU, Bandello F, Loewenstein A. Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin Ophthalmol. 2015;9:1321–35.
Campochiaro PA, Marcus DM, Awh CC, Regillo C, Adamis AP, Bantseev V, et al. The port delivery system with ranibizumab for neovascular age-related macular degeneration: results from the randomized phase 2 ladder clinical trial. Ophthalmology. 2019;126:1141–54.
Fong DS, Girach A, Boney A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: a literature review. Retina. 2007;27:816–24.
Elman MJ, Ayala A, Bressler NM, Browning D, Flaxel CJ, Glassman AR, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology. 2015;122:375–81.
Reichle ML. Complications of intravitreal steroid injections. Optometry. 2005;76:450–60.
Heier JS, Antoszyk AN, Pavan PR, Leff SR, Rosenfeld PJ, Ciulla TA, et al. Ranibizumab for treatment of neovascular age-related macular degeneration: a phase I/II multicenter, controlled, multidose study. Ophthalmology. 2006;113(633):e631–4.
Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113:363–72 e365.
Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2019.
Dugel PU, Jaffe GJ, Sallstig P, Warburton J, Weichselberger A, Wieland M, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology. 2017;124:1296–304.
Stahl A, Stumpp MT, Schlegel A, Ekawardhani S, Lehrling C, Martin G, et al. Highly potent VEGF-A-antagonistic DARPins as anti-angiogenic agents for topical and intravitreal applications. Angiogenesis. 2013;16:101–11.
Smithwick E, Stewart MW. Designed ankyrin repeat proteins: a look at their evolving use in medicine with a focus on the treatment of chorioretinal vascular disorders. Antiinflamm Antiallergy Agents Med Chem. 2017;16:33–45.
Hussain RM, Ciulla TA. Emerging vascular endothelial growth factor antagonists to treat neovascular age-related macular degeneration. Expert Opin Emerg Drugs. 2017;22:235–46.
Jaffe GJ, Ciulla TA, Ciardella AP, Devin F, Dugel PU, Eandi CM, et al. Dual antagonism of PDGF and VEGF in neovascular age-related macular degeneration: a phase IIb, multicenter, randomized controlled trial. Ophthalmology. 2017;124:224–34.
Yaspan BL, Williams DF, Holz FG, Regillo CD, Li Z, Dressen A, et al. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med. 2017;9.
Dolgin E. Age-related macular degeneration foils drugmakers. Nat Biotechnol. 2017;35:1000–1.
Rosenfeld PJ, Feuer WJ. Lessons from recent phase III trial failures: don't sesign phase III trials based on retrospective subgroup analyses from phase II trials. Ophthalmology. 2018;125:1488–91.
Pocock SJ, Stone GW. The primary outcome fails - what next? N Engl J Med. 2016;375:861–70.
PanOptica Inc.
Thakur A, Scheinman RI, Rao VR, Kompella UB. Pazopanib, a multitargeted tyrosine kinase inhibitor, reduces diabetic retinal vascular leukostasis and leakage. Microvasc Res. 2011;82:346–50.
Samiy N. Gene therapy for retinal diseases. J Ophthalmic Vis Res. 2014;9:506–9.
MacLaren RE, Bennett J, Schwartz SD. Gene therapy and stem cell transplantation in retinal disease: the new frontier. Ophthalmology. 2016;123:S98–S106.
McGill TJ, Bohana-Kashtan O, Stoddard JW, Andrews MD, Pandit N, Rosenberg-Belmaker LR, et al. Long-term efficacy of GMP grade xeno-free hESC-derived RPE cells following transplantation. Transl Vis Sci Technol. 2017;6:17.
Ben M'Barek K, Habeler W, Plancheron A, Jarraya M, Regent F, Terray A, et al. Human ESC-derived retinal epithelial cell sheets potentiate rescue of photoreceptor cell loss in rats with retinal degeneration. Sci Transl Med. 2017;9.
Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Dang W, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med. 2018;10.
Byrne LC. What's old is new again: autologous stem cell transplant for AMD. Sci Transl Med. 2017;9.
Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038–46.
Holmes D. Retinal repair: visions of the future. Nature. 2018;561:S1.
Abbasi J. Stem cell implants for age-related macular degeneration. JAMA. 2018;319:2263.
Sharma R, Khristov V, Rising A, Jha BS, Dejene R, Hotaling N, et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci Transl Med. 2019;11.
McGill TJ, Stoddard J, Renner LM, Messaoudi I, Bharti K, Mitalipov S, et al. Allogeneic iPSC-derived RPE cell graft failure following transplantation into the subretinal space in nonhuman primates. Invest Ophthalmol Vis Sci. 2018;59:1374–83.
Nguyen PK, Sarkar B, Siddiqui Z, McGowan M, Iglesias-Montoro P, Rachapudi S, et al. Self-assembly of an anti-angiogenic nanofibrous peptide hydrogel. ACS Appl Bio Mater. 2018;1:865–70.
Bhise NS, Shmueli RB, Sunshine JC, Tzeng SY, Green JJ. Drug delivery strategies for therapeutic angiogenesis and antiangiogenesis. Expert Opin Drug Deliv. 2011;8:485–504.
Dong H, Paramonov SE, Aulisa L, Bakota EL, Hartgerink JD. Self-assembly of multidomain peptides: balancing molecular frustration controls conformation and nanostructure. J Am Chem Soc. 2007;129:12468–72.
Aulisa L, Dong H, Hartgerink JD. Self-assembly of multidomain peptides: sequence variation allows control over cross-linking and viscoelasticity. Biomacromolecules. 2009;10:2694–8.
Kumar VA, Taylor NL, Shi S, Wang BK, Jalan AA, Kang MK, et al. Highly angiogenic peptide nanofibers. ACS Nano. 2015;9:860–8.
Moore AN, Hartgerink JD. Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Acc Chem Res. 2017;50:714–22.
Moore AN, Lopez Silva TL, Carrejo NC, Origel Marmolejo CA, Li IC, Hartgerink JD. Nanofibrous peptide hydrogel elicits angiogenesis and neurogenesis without drugs, proteins, or cells. Biomaterials. 2018;161:154–63.
Sarkar B, Siddiqui Z, Nguyen PK, Dube N, Fu W, Park S, et al. Membrane-disrupting nanofibrous peptide hydrogels. ACS Biomat Sci Eng. 2019. https://doi.org/10.1021/acsbiomaterials.1029b00967.
Kumar VA, Liu Q, Wickremasinghe NC, Shi S, Cornwright TT, Deng Y, et al. Treatment of hind limb ischemia using angiogenic peptide nanofibers. Biomaterials. 2016;98:113–9.
Hitscherich P, Nguyen PK, Kannan A, Chirayath A, Anur S, Sarkar B, et al. Injectable self-assembling peptide hydrogels for tissue writing and embryonic stem cell culture. J Biomed Nanotechnol. 2018;14:802–7.
Nguyen PK, Gao W, Patel SD, Siddiqui Z, Weiner S, Shimizu E, et al. Self-assembly of a dentinogenic peptide hydrogel. ACS Omega. 2018;3:5980–7.
Kumar VA, Taylor NL, Shi S, Wickremasinghe NC, D'Souza RN, Hartgerink JD. Self-assembling multidomain peptides tailor biological responses through biphasic release. Biomaterials. 2015;52:71–8.
Kumar VA, Shi S, Wang BK, Li IC, Jalan AA, Sarkar B, et al. Drug-triggered and cross-linked self-assembling nanofibrous hydrogels. J Am Chem Soc. 2015;137:4823–30.
Li IC, Moore AN, Hartgerink JD. "Missing tooth" multidomain peptide nanofibers for delivery of small molecule drugs. Biomacromolecules. 2016;17:2087–95.
Leach DG, Dharmaraj N, Piotrowski SL, Lopez-Silva TL, Lei YL, Sikora AG, et al. STINGel: controlled release of a cyclic dinucleotide for enhanced cancer immunotherapy. Biomaterials. 2018;163:67–75.
Sarkar B, Nguyen PK, Gao W, Dondapati A, Siddiqui Z, Kumar VA. Angiogenic self-assembling peptide scaffolds for functional tissue regeneration. Biomacromolecules. 2018;19:3597–611.
Webber MJ, Tongers J, Newcomb CJ, Marquardt KT, Bauersachs J, Losordo DW, et al. Supramolecular nanostructures that mimic VEGF as a strategy for ischemic tissue repair. Proc Natl Acad Sci U S A. 2011;108:13438–43.
Li R, Xu J, Wong DSH, Li J, Zhao P, Bian L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/beta-catenin signaling. Biomaterials. 2017;145:33–43.
Rosca EV, Koskimaki JE, Rivera CG, Pandey NB, Tamiz AP, Popel AS. Anti-angiogenic peptides for cancer therapeutics. Curr Pharm Biotechnol. 2011;12:1101–16.
Davidson DJ, Haskell C, Majest S, Kherzai A, Egan DA, Walter KA, et al. Kringle 5 of human plasminogen induces apoptosis of endothelial and tumor cells through surface-expressed glucose-regulated protein 78. Cancer Res. 2005;65:4663–72.
Zhang SX, Sima J, Wang JJ, Shao C, Fant J, Ma JX. Systemic and periocular deliveries of plasminogen kringle 5 reduce vascular leakage in rat models of oxygen-induced retinopathy and diabetes. Curr Eye Res. 2005;30:681–9.
Nguyen TM, Subramanian IV, Kelekar A, Ramakrishnan S. Kringle 5 of human plasminogen, an angiogenesis inhibitor, induces both autophagy and apoptotic death in endothelial cells. Blood. 2007;109:4793–802.
Yi ZF, Cho SG, Zhao H, Wu YY, Luo J, Li D, et al. A novel peptide from human apolipoprotein(a) inhibits angiogenesis and tumor growth by targeting c-Src phosphorylation in VEGF-induced human umbilical endothelial cells. Int J Cancer. 2009;124:843–52.
Ponce ML, Hibino S, Lebioda AM, Mochizuki M, Nomizu M, Kleinman HK. Identification of a potent peptide antagonist to an active laminin-1 sequence that blocks angiogenesis and tumor growth. Cancer Res. 2003;63:5060–4.
Dixelius J, Olsson AK, Thulin A, Lee C, Johansson I, Claesson-Welsh L. Minimal active domain and mechanism of action of the angiogenesis inhibitor histidine-rich glycoprotein. Cancer Res. 2006;66:2089–97.
Cai X, McGinnis JF. Diabetic retinopathy: animal models, therapies, and perspectives. J Diabetes Res. 2016;2016:3789217.
Olivares AM, Althoff K, Chen GF, Wu S, Morrisson MA, DeAngelis MM, et al. Animal models of diabetic retinopathy. Curr Diab Rep. 2017;17:93.
Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, et al. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26:270–1.
Khayat M, Lois N, Williams M, Stitt AW. Animal models of retinal vein occlusion" Investig Opthalmol Vis Sci. 2017;58.
Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol Asp Med. 2012;33:487–509.
Lai AK, Lo AC. Animal models of diabetic retinopathy: summary and comparison. J Diabetes Res. 2013;2013:106594.
Fletcher EL, Jobling AI, Greferath U, Mills SA, Waugh M, Ho T, et al. Studying age-related macular degeneration using animal models. Optom Vis Sci. 2014;91:878–86.
Singh RP, Habbu KA, Bedi R, Silva FQ, Ehlers JP, Schachat AP, et al. A retrospective study of the influence of the vitreomacular interface on macular oedema secondary to retinal vein occlusion. Br J Ophthalmol. 2017;101:1340–5.
Young JM, Wai KM, Silva FQ, Conti FF, Srivastava SK, Ehlers JP, et al. Long-term outcomes of anti-VEGF therapy in patients with macular edema secondary to retinal vein occlusion. J VitreoRetinal Dis. 2017;1:298–304.
Au A, Parikh VS, Singh RP, Ehlers JP, Yuan A, Rachitskaya AV, et al. Comparison of anti-VEGF therapies on fibrovascular pigment epithelial detachments in age-related macular degeneration. Br J Ophthalmol. 2017;101:970–5.
Wai KM, Khan M, Srivastava S, Rachitskaya A, Silva FQ, Deasy R, et al. Impact of initial visual acuity on anti-VEGF treatment outcomes in patients with macular oedema secondary to retinal vein occlusions in routine clinical practice. Br J Ophthalmol. 2017;101:574–9.
Bell BA, Yuan A, Dicicco RM, Fogerty J, Lessieur EM, Perkins BD. The adult zebrafish retina: in vivo optical sectioning with confocal scanning laser ophthalmoscopy and spectral-domain optical coherence tomography. Exp Eye Res. 2016;153:65–78.
Edmond M, Yuan A, Bell BA, Sharma A, DiCicco RM, Tucker L, et al. The feasibility of spectral-domain optical coherence tomography grading of anterior chamber inflammation in a rabbit model of anterior uveitis. Invest Ophthalmol Vis Sci. 2016;57:OCT184–8.
Yuan A, Ahmad BU, Xu D, Singh RP, Kaiser PK, Martin DF, et al. Comparison of intravitreal ranibizumab and bevacizumab for the treatment of macular edema secondary to retinal vein occlusion. Int J Ophthalmol. 2014;7:86–91.
Yuan A, Singh RP. Radiation maculopathy treated with ranibizumab. J Clin Exp Ophthalmol. 2011;02.
Yuan A, Singh R. Ranibizumab for the treatment of macular edema following retinal vein occlusion. Clin Investig. 2011;1:1445–54.
Bell BA, Xie J, Yuan A, Kaul C, Hollyfield JG, Anand-Apte B. Retinal vasculature of adult zebrafish: in vivo imaging using confocal scanning laser ophthalmoscopy. Exp Eye Res. 2014;129:107–18.
Pierro L, Zampedri E, Milani P, Gagliardi M, Isola V, Pece A. Spectral domain OCT versus time domain OCT in the evaluation of macular features related to wet age-related macular degeneration. Clin Ophthalmol. 2012;6:219–23.
Rachitskaya AV, Ehlers JP, Yuan A. Intraoperative OCT of a retinal tack. Ophthalmol Retina. 2017;1:420.
Rachitskaya AV, Yuan A, Singh RP, Sears JE, Schachat AP. Optical coherence tomography of outer retinal holes in senile retinoschisis and schisis-detachment. Br J Ophthalmol. 2017;101:445–8.
Rachitskaya AV, Yuan A, Marino MJ, Reese J, Ehlers JP. Intraoperative OCT imaging of the Argus II retinal prosthesis system. Ophthalmic Surg Lasers Imaging Retina. 2016;47:999–1003.
Ehlers JP, Petkovsek DS, Yuan A, Singh RP, Srivastava SK. Intrasurgical assessment of subretinal tPA injection for submacular hemorrhage in the PIONEER study utilizing intraoperative OCT. Ophthalmic Surg Lasers Imaging Retina. 2015;46:327–32.
DiCicco RM, Bell BA, Kaul C, Hollyfield JG, Anand-Apte B, Perkins BD, et al. Retinal regeneration following OCT-guided laser injury in zebrafish. Investig Opthalmol Vis Sci. 2014;55.
Ehlers JP, Yuan A, Kaiser PK, Dhoot D, Sears JE, Martin DF, et al. Trans-tamponade optical coherence tomography: postoperative imaging in gas-filled eyes. Retina. 2013;33:1172–8.
Xu D, Yuan A, Kaiser PK, Srivastava SK, Singh RP, Sears JE, et al. A novel segmentation algorithm for volumetric analysis of macular hole boundaries identified with optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54:163–9.
Dhoot DS, Huo S, Yuan A, Xu D, Srivistava S, Ehlers JP, et al. Evaluation of choroidal thickness in retinitis pigmentosa using enhanced depth imaging optical coherence tomography. Br J Ophthalmol. 2013;97:66–9.
Roth BM, Yuan A, Ehlers JP. Retinal and choroidal findings in oxalate retinopathy using EDI-OCT. Ophthalmic Surg Lasers Imaging. 2012;43:S142–4.
Yuan A, Ehlers JP. Crystalline retinopathy from primary hyperoxaluria. Retina. 2012;32:1994–5.
Yuan A, Kaines A, Jain A, Reddy S, Schwartz SD, Sarraf D. Ultra-wide-field and autofluorescence imaging of choroidal dystrophies. Ophthalmic Surg Lasers Imaging. 2010;41(Online):e1–5.
DeFrancesco L. Three deaths sink Affymax. Nat Biotechnol. 2013;31:270.
Acknowledgments
We would like to thank Dr. Alex Yuan (Cleveland Clinic) for helpful discussions and feedback.
Funding
This work was supported by grant NIH R15 EY029504, NSF IIP 1903617, the NJIT Undergraduate Research and Innovation (URI) Program, and NJIT Startup funds (to V.A.K.).
Author information
Authors and Affiliations
Contributions
The review was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
V.A.K. has equity interests in start-up companies attempting to translate peptides from peptide-based technological platform.
Declaration of informed consent and animal studies
Not applicable for this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarkar, B., Siddiqui, Z., Kim, K.K. et al. Implantable anti-angiogenic scaffolds for treatment of neovascular ocular pathologies. Drug Deliv. and Transl. Res. 10, 1191–1202 (2020). https://doi.org/10.1007/s13346-020-00753-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00753-0