Abstract
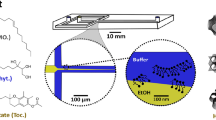
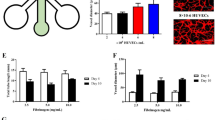
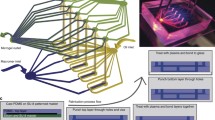
Nonlamellar lipid-based liquid crystalline (LLC) nanoparticles possessing different internal nanostructures, specifically the 3D-ordered cubosomes (V2 phase) and the 2D-ordered hexosomes (H2 phase), are of increasing interest as drug delivery systems. To facilitate their development, it is important that we understand their interactions with healthy human umbilical vein endothelial cells (HUVECs). To this end, a 3D cells-in-a-tube model that recapitulates the basic morphology (i.e. tubular lumen) and in vivo microenvironment (i.e. physiological shear stress) of blood vessels was employed as a biomimetic testing platform, and the bio-nanoparticle interactions were compared with that of the conventional 2D planar cell culture. Confocal microscopy imaging revealed internalisation of the nanoparticles into HUVECs within 2 h and that the nanoparticle-cell interactions of cubosomes and hexosomes were not significantly different from one another. Low fluid shear stress conditions (i.e. venous simulation at 0.8 dynes/cm2) were shown to impose subtle effects on the degree of nanoparticle-cell interactions as compared with the static 2D culture. The unexpected similarity of cellular interactions between cubosomes and hexosomes was clarified via a real-time phase behaviour analysis using the synchrotron-based small-angle X-ray scattering (SAXS) technique. When the nanoparticles came into contact with HUVECs under circulating conditions, the cubosomes gradually evolved into hexosomes (within 16 min). In contrast, the hexosomes retained their original internal structure with minimal changes to the lattice parameters. This study highlights the need to couple cellular studies with high-resolution analytics such as time-resolved SAXS analysis to ensure that particle structures are verified in situ, enabling accurate interpretation of the dynamics of cellular interactions and potential bio-induced changes of particles intended for biomedical applications.

Graphical abstract








Similar content being viewed by others
References
Chow EKH, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med. 2013;5:216.
Fong WK, Negrini R, Vallooran JJ, Mezzenga R, Boyd BJ. Responsive self-assembled nanostructured lipid systems for drug delivery and diagnostics. J Colloid Interface Sci. 2016;484:320–39.
Ruenraroengsak P, Cook JM, Florence AT. Nanosystem drug targeting: facing up to complex realities. J Control Release. 2010;141(3):265–76.
Medina-Reyes EI, García-Viacobo D, Carrero-Martínez FA, Chirino YI. Applications and risks of nanomaterials used in regenerative medicine, delivery systems, theranostics, and therapy. Crit Rev Ther Drug Carrier Syst. 2017;34:35–61.
Boyd BJ, Dong YD, Rades T. Nonlamellar liquid crystalline nanostructured particles: advances in materials and structure determination. J Liposome Res. 2009;19:12–28.
van't Hag L, Gras SL, Conn CE, Drummond CJ. Lyotropic liquid crystal engineering moving beyond binary compositional space-ordered nanostructured amphiphile self-assembly materials by design. Chem Soc Rev. 2017;46:2705–31.
Chen Y, Ma P, Gui S. Cubic and hexagonal liquid crystals as drug delivery systems. Biomed Res Int. 2014;2014:815981.
Fong WK, Hanley TL, Thierry B, Tilley A, Kirby N, Waddington LJ, et al. Understanding the photothermal heating effect in non-lamellar liquid crystalline systems, and the design of new mixed lipid systems for photothermal on-demand drug delivery. Phys Chem Chem Phys. 2014;16:24936–53.
Riedinger A, Guardia P, Curcio A, Garcia MA, Cingolani R, Manna L, et al. Subnanometer local temperature probing and remotely controlled drug release based on azo-functionalized iron oxide nanoparticles. Nano Lett. 2013;13:2399–406.
Gessner A, Waicz R, Lieske A, Pauke BR, Mader K, Muller RH. Nanoparticles with decreasing surface hydrophobicities: influence on plasma protein adsorption. Int J Pharm. 2000;196:245–9.
Dong YD, Larson I, Hanley T, Boyd BJ. Bulk and dispersed aqueous phase behavior of phytantriol: effect of vitamin E acetate and F127 polymer on liquid crystal nanostructure. Langmuir. 2006;22:9512–8.
Sabliov CM, Chen H, Yada RY. Nanotechnology and functional foods: effective delivery of bioactive ingredients: Wiley Blackwell; 2015.
Godoy-Gallardo M, Ek PK, Jansman MMT, Wohl BM, Hosta-Rigau L. Interaction between drug delivery vehicles and cells under the effect of shear stress. Biomicrofluidics. 2015;9:052605.
Guerzoni LPB, Nicolas V, Angelova A. In vitro modulation of TrkB receptor signaling upon sequential delivery of curcumin-DHA loaded carriers towards promoting neuronal survival. Pharm Res. 2017;34:492–505.
Rakotoarisoa M, Angelov B, Garamus VM, Angelova A. Curcumin- and fish oil-loaded spongosome and cubosome nanoparticles with neuroprotective potential against H2O2-induced oxidative stress in differentiated human SH-SY5Y cells. ACS Omega. 2019;4:3061–73.
Feliu N, Sun X, Alvarez Puebla RA, Parak WJ. Quantitative particle-cell interaction: some basic physicochemical pitfalls. Langmuir. 2017;33:6639–46.
Woo K, Dutta AK, Patel V, Kresge C, Feranchak AP. Fluid flow induces mechanosensitive ATP release, calcium signalling and Cl-transport in biliary epithelial cells through a PKCζ-dependent pathway. J Physiol. 2008;586:2779–98.
Gupta R, Truong L, Bear D, Chafik D, Modafferi E, Hung CT. Shear stress alters the expression of myelin-associated glycoprotein (MAG) and myelin basic protein (MBP) in Schwann cells. J Orthop Res. 2005;23:1232–9.
Anton D, Burckel H, Josset E, Noel G. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16:5517–27.
Weber D, Torger B, Richter K, Nessling M, Momburg F, Woltmann B, et al. Interaction of poly(L-lysine)/polysaccharide complex nanoparticles with human vascular endothelial cells. Nanomaterials. 2018;8.
Voigt J, Christensen J, Shastri VP. Differential uptake of nanoparticles by endothelial cells through polyelectrolytes with affinity for caveolae. P Natl A Sci USA. 2014;111:2942.
Kirby NM, Mudie ST, Hawley AM, Cookson DJ, Mertens HDT, Cowieson N, et al. A low-background-intensity focusing small-angle X-ray scattering undulator beamline. J Appl Crystallogr. 2013;46:1670–80.
Tan A, Lam YY, Pacot O, Hawley A, Boyd BJ. Probing cell-nanoparticle (cubosome) interactions at the endothelial interface: do tissue dimension and flow matter? Biomater Sci. 2019;7:3460–70.
Tan A, Fujisawa K, Yukawa Y, Matsunaga YT. Bottom-up fabrication of artery-mimicking tubular co-cultures in collagen-based microchannel scaffolds. Biomater Sci. 2016;4:1503–14.
Mezzenga R, Meyer C, Servais C, Romoscanu AI, Sagalowicz L, Hayward RC. Shear rheology of lyotropic liquid crystals: a case study. Langmuir. 2005;21:3322–33.
Fong C, Weerawardena A, Sagnella SM, Mulet X, Waddington L, Krodkiewska I, et al. Monodisperse nonionic phytanyl ethylene oxide surfactants: high throughput lyotropic liquid crystalline phase determination and the formation of liposomes, hexosomes and cubosomes. Soft Matter. 2010;6:4727–41.
Angelov B, Garamus VM, Drechsler M, Angelova A. Structural analysis of nanoparticulate carriers for encapsulation of macromolecular drugs. J Mol Liq. 2017;235:83–9.
Angelova A, Drechsler M, Garamus VM, Angelov B. Pep-lipid cubosomes and vesicles compartmentalized by micelles from self-assembly of multiple neuroprotective building blocks including a large peptide hormone PACAP-DHA. ChemNanoMat. 2019;5:1381–9.
Zhang S, Gao H, Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9:8655–71.
Mat Azmi ID, Wu L, Wibroe PP, Nilsson C, Østergaard J, Stürup S, et al. Modulatory effect of human plasma on the internal nanostructure and size characteristics of liquid-crystalline nanocarriers. Langmuir. 2015;31:5042–9.
Hinton TM, Grusche F, Acharya D, Shukla R, Bansal V, Waddington LJ, et al. Bicontinuous cubic phase nanoparticle lipid chemistry affects toxicity in cultured cells. Toxicol Res. 2014;3:11–22.
Tran N, Mulet X, Hawley AM, Hinton TM, Mudie ST, Muir BW, et al. Nanostructure and cytotoxicity of self-assembled monoolein-capric acid lyotropic liquid crystalline nanoparticles. RSC Adv. 2015;5:26785–95.
Tan A, Hong L, Du JD, Boyd BJ. Self-assembled nanostructured lipid systems: is there a link between structure and cytotoxicity? Adv Sci. 2019;6:1801223.
Acknowledgements
Mr. Cameron J. Nowell assisted the confocal microscopy imaging and analysis at the imaging, FACS, and Analysis Core, Monash University. The cryo-TEM images were taken at the Bio21 Institute with the assistance of Ms. Xiaohan Sun (Monash PhD candidate). The SAXS experiments were undertaken on the SAXS/WAXS beamline at the ANSTO Australian Synchrotron, Victoria.
Funding
This project is funded by the Australian Research Council (ARC) Centre of Excellence in Convergent Bio-Nano Science and Technology. B.J.B. was the recipient of an Australian Research Council Future Fellowship. Y.Y.L. was supported by a Monash University Jubilee Honours Scholarship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 318 kb)
Rights and permissions
About this article
Cite this article
Lam, Y.Y., Hawley, A., Tan, A. et al. Coupling in vitro cell culture with synchrotron SAXS to understand the bio-interaction of lipid-based liquid crystalline nanoparticles with vascular endothelial cells. Drug Deliv. and Transl. Res. 10, 610–620 (2020). https://doi.org/10.1007/s13346-020-00718-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00718-3