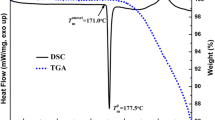
Abstract
In this study, using the melt-adsorption method, we developed sustained-release microparticles containing the potent drug, tamsulosin HCl, for use as orally disintegrating tablets. A high-speed kneading granulator was used, enabling temperature modulation and uniform material distribution. A lipid and ethylcellulose suspension (Surelease®) was applied to retard drug release, and magnesium aluminometasilicate (Neusilin®) was used as adsorbent. Among various lipid candidates for melt-adsorption, beeswax and glyceryl behenate were selected due to their high mechanical strength. Hot stage microscopy and powder X-ray diffraction analysis results showed compatibility between tamsulosin HCl and both lipids. Characteristic adsorption behavior was observed depending on the physicochemical properties of each composition. Especially, the specific surface area of Neusilin® decreased with increasing amounts of Surelease®, attributed to the pore-covering effect of Surelease®, which significantly increased the size of the microparticles after the lipid adsorption. For a Surelease®-to-beeswax ratio 1:50, both the desired particle size distribution and low burst release were achieved. Furthermore, the orally disintegrating tablet containing optimized microparticles had acceptable tablet hardness and rapid disintegration. Herein, the feasibility of melt-adsorption for the preparation of sustained-release microparticles was well demonstrated. With its convenience and efficiency, the proposed method is a promising alternative to conventional methods, which are relatively difficult and time consuming.










Similar content being viewed by others
References
Dungarwal UN, Patil SB. Development of orodispersible tablets of taste masked rizatriptan benzoate using hydroxypropyl β cyclodextrin. J Pharm Investig. 2016;46(6):537–45. https://doi.org/10.1007/s40005-016-0240-5.
Venkatesh GM, Stevens PJ, Lai J-W. Development of orally disintegrating tablets comprising controlled-release multiparticulate beads. Drug Dev Ind Pharm. 2012;38(12):1428–40. https://doi.org/10.3109/03639045.2011.653365.
S-i K, Uchida S, Kanada K, Namiki N. Effect of granule properties on rough mouth feel and palatability of orally disintegrating tablets. Int J Pharm. 2015;484(1):156–62.
Kim J-Y, Hwang K-M, Park C-W, Rhee Y-S, Park E-S. Organic-aqueous crossover coating process for the desmopressin orally disintegrating microparticles. Drug Dev Ind Pharm. 2015;41(2):292–9. https://doi.org/10.3109/03639045.2013.858742.
Kinoshita M, Baba K, Nagayasu A, Yamabe K, Shimooka T, Yi T, et al. Improvement of solubility and oral bioavailability of a poorly water-soluble drug, TAS-301, by its melt-adsorption on a porous calcium silicate. J Pharm Sci. 2002;91(2):362–70. https://doi.org/10.1002/jps.10026.
Lainé A-L, Price D, Davis J, Roberts D, Hudson R, Back K, et al. Enhanced oral delivery of celecoxib via the development of a supersaturable amorphous formulation utilising mesoporous silica and co-loaded HPMCAS. Int J Pharm. 2016;512(1):118–25. https://doi.org/10.1016/j.ijpharm.2016.08.034.
Cha K-H, Cho K-J, Kim M-S, Kim J-S, Park HJ, Park J, et al. Enhancement of the dissolution rate and bioavailability of fenofibrate by a melt-adsorption method using supercritical carbon dioxide. Int J Nanomedicine. 2012;7:5565.
Dangre PV, Gilhotra RM, Dhole SN. Formulation and development of solid self micro-emulsifying drug delivery system (S-SMEDDS) containing chlorthalidone for improvement of dissolution. J Pharm Investig. 2016;46(7):633–44. https://doi.org/10.1007/s40005-016-0243-2.
Yan X, He H, Meng J, Zhang C, Hong M, Tang X. Preparation of lipid aspirin sustained-release pellets by solvent-free extrusion/spheronization and an investigation of their stability. Drug Dev Ind Pharm. 2012;38(10):1221–9. https://doi.org/10.3109/03639045.2011.645829.
Jannin V, Cuppok Y. Hot-melt coating with lipid excipients. Int J Pharm. 2013;457(2):480–7. https://doi.org/10.1016/j.ijpharm.2012.10.026.
Shah A, Serajuddin AT. Conversion of solid dispersion prepared by acid–base interaction into free-flowing and tabletable powder by using Neusilin® US2. Int J Pharm. 2015;484(1):172–80. https://doi.org/10.1016/j.ijpharm.2015.02.060.
Hentzschel C, Alnaief M, Smirnova I, Sakmann A, Leopold C. Enhancement of griseofulvin release from liquisolid compacts. Eur J Pharm Biopharm. 2012;80(1):130–5. https://doi.org/10.1016/j.ejpb.2011.08.001.
Zhao X, Zhou Y, Potharaju S, Lou H, Sun H, Brunson E, et al. Development of a self micro-emulsifying tablet of cyclosporine-A by the liquisolid compact technique. Int J Pharm Sci Res. 2011;2(9):2299.
Hentzschel CM, Sakmann A, Leopold CS. Suitability of various excipients as carrier and coating materials for liquisolid compacts. Drug Dev Ind Pharm. 2011;37(10):1200–7. https://doi.org/10.3109/03639045.2011.564184.
Ohno T, Shimizu T, Kato S, Hayashi H, Hirai S. Effect of tamsulosin hydrochloride on sympathetic hyperactivity in amyotrophic lateral sclerosis. Auton Neurosci. 2001;88(1):94–8. https://doi.org/10.1016/S1566-0702(01)00217-X.
Barry MJ, Fowler FJ, O'leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 2017;197(2):S189–S97. https://doi.org/10.1016/j.juro.2016.10.071.
Vercruysse J, Peeters E, Fonteyne M, Cappuyns P, Delaet U, Van Assche I, et al. Use of a continuous twin screw granulation and drying system during formulation development and process optimization. Eur J Pharm Biopharm. 2015;89:239–47. https://doi.org/10.1016/j.ejpb.2014.12.017.
Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23.
Aburahma MH, Badr-Eldin SM. Compritol 888 ATO: a multifunctional lipid excipient in drug delivery systems and nanopharmaceuticals. Expert Opin Drug Deliv. 2014;11(12):1865–83. https://doi.org/10.1517/17425247.2014.935335.
Bonvehi JS, Bermejo FO. Detection of adulterated commercial Spanish beeswax. Food Chem. 2012;132(1):642–8. https://doi.org/10.1016/j.foodchem.2011.10.104.
Maeda A, Shinoda T, Ito N, Baba K, Oku N, Mizumoto T. Evaluating tamsulosin hydrochloride-released microparticles prepared using single-step matrix coating. Int J Pharm. 2011;408(1):84–90. https://doi.org/10.1016/j.ijpharm.2011.01.053.
Sokolsky-Papkov M, Domb AJ. Stereoisomeric effect on in vitro drug release from injectable poly (lactic acid co castor oil) polyesters. Polym Adv Technol. 2008;19(6):671–9. https://doi.org/10.1002/pat.1140.
Gupta MK, Goldman D, Bogner RH, Tseng Y-C. Enhanced drug dissolution and bulk properties of solid dispersions granulated with a surface adsorbent. Pharm Dev Technol. 2001;6(4):563–72. https://doi.org/10.1081/PDT-120000294.
Vadher AH, Parikh JR, Parikh RH, Solanki AB. Preparation and characterization of co-grinded mixtures of aceclofenac and Neusilin US 2 for dissolution enhancement of aceclofenac. AAPS PharmSciTech. 2009;10(2):606–14. https://doi.org/10.1208/s12249-009-9221-6.
Mura P, Valleri M, Cirri M, Mennini N. New solid self-microemulsifying systems to enhance dissolution rate of poorly water soluble drugs. Pharm Dev Technol. 2012;17(3):277–84. https://doi.org/10.3109/10837450.2010.535825.
Williams HD, Van Speybroeck M, Augustijns P, Porter CJ. Lipid-based formulations solidified via adsorption onto the mesoporous carrier Neusilin® US2: effect of drug type and formulation composition on in vitro pharmaceutical performance. J Pharm Sci. 2014;103(6):1734–46. https://doi.org/10.1002/jps.23970.
Kutza C, Metz H, Kutza J, Syrowatka F, Mäder K. Toward a detailed characterization of oil adsorbates as “solid liquids”. Eur J Pharm Biopharm. 2013;84(1):172–82. https://doi.org/10.1016/j.ejpb.2012.12.008.
Wei Q, Keck CM, Müller RH. Preparation and tableting of long-term stable amorphous rutin using porous silica. Eur J Pharm Biopharm. 2017;113:97–107. https://doi.org/10.1016/j.ejpb.2016.11.009.
Rajabi-Siahboomi AR, Mehta RY, Ambudkar V, Dias V, Tiwari S. Ethylcellulose applications in multiparticulate systems. Multiparticulate Drug Delivery. Springer; 2017. p. 267–99.
Melegari C, Bertoni S, Genovesi A, Hughes K, Rajabi-Siahboomi AR, Passerini N, et al. Ethylcellulose film coating of guaifenesin-loaded pellets: a comprehensive evaluation of the manufacturing process to prevent drug migration. Eur J Pharm Biopharm. 2016;100:15–26. https://doi.org/10.1016/j.ejpb.2015.12.001.
Kim J-Y, An S-H, Rhee Y-S, Park C-W, Park E-S. A comparative study between spray-drying and fluidized bed coating processes for the preparation of pramipexole controlled-release microparticles for orally disintegrating tablets. Dry Technol. 2014;32(8):935–45. https://doi.org/10.1080/07373937.2013.875562.
Klinger GH, Ghali ES, Porter SC, Schwartz JB. Formulation of controlled release matrices by granulation with a polymer dispersion. Drug Dev Ind Pharm. 1990;16(9):1473–90. https://doi.org/10.3109/03639049009074378.
Tsai T, San Y-P, Ho H-O, Wu J-S, Sheu M-T. Film-forming polymer-granulated excipients as the matrix materials for controlled release dosage forms. J Control Release. 1998;51(2):289–99. https://doi.org/10.1016/S0168-3659(97)00183-1.
Khan GM, Zhu JB. Ibuprofen release kinetics from controlled-release tablets granulated with aqueous polymeric dispersion of ethylcellulose II: influence of several parameters and coexcipients. J Control Release. 1998;56(1):127–34. https://doi.org/10.1016/S0168-3659(98)00080-7.
Chaibva FA, Walker RB. The use of response surface methodology for the formulation and optimization of salbutamol sulfate hydrophilic matrix sustained release tablets. Pharm Dev Technol. 2012;17(5):594–606. https://doi.org/10.3109/10837450.2011.557731.
Afrasiabi Garekani H, Faghihnia Torshizi M, Sadeghi F. Surelease as granulating liquid in preparation of sustained release matrices of ethylcellulose and theophylline. Drug Dev Ind Pharm. 2015;41(10):1655–60. https://doi.org/10.3109/03639045.2014.983929.
McConnell EL, Tutas J, Mohamed MAM, Banning D, Basit AW. Colonic drug delivery using amylose films: the role of aqueous ethylcellulose dispersions in controlling drug release. Cellulose. 2007;14(1):25–34.
Kheradmandnia S, Vasheghani-Farahani E, Nosrati M, Atyabi F. Preparation and characterization of ketoprofen-loaded solid lipid nanoparticles made from beeswax and carnauba wax. Nanomedicine. 2010;6(6):753–9. https://doi.org/10.1016/j.nano.2010.06.003.
Jeong KH, Woo HS, Kim CJ, Lee KH, Jeon JY, Lee SY, et al. Formulation of a modified-release pregabalin tablet using hot-melt coating with glyceryl behenate. Int J Pharm. 2015;495(1):1–8. https://doi.org/10.1016/j.ijpharm.2015.08.057.
Vergote G, Kiekens F, Vervaet C, Remon JP. Wax beads as cushioning agents during the compression of coated diltiazem pellets. Eur J Pharm Sci. 2002;17(3):145–51. https://doi.org/10.1016/S0928-0987(02)00164-1.
Al-Khattawi A, Mohammed AR. Challenges and emerging solutions in the development of compressed orally disintegrating tablets. Expert Opin Drug Discovery. 2014;9(10):1109–20. https://doi.org/10.1517/17460441.2014.941802.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (grant number NRF-2016R1A2B4007101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cho, CH., Min, JH., Hwang, KM. et al. Development of sustained-release microparticles containing tamsulosin HCl for orally disintegrating tablet using melt-adsorption method. Drug Deliv. and Transl. Res. 8, 552–564 (2018). https://doi.org/10.1007/s13346-018-0477-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-0477-9